Mirolabrichthys Herre, 1927
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5092.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:A546CCCB-6072-434B-B366-1AFB1BE20CD8 |
|
DOI |
https://doi.org/10.5281/zenodo.5883163 |
|
persistent identifier |
https://treatment.plazi.org/id/1B7A87C4-FFB0-FFC0-B7AB-1A06FC2DFCDA |
|
treatment provided by |
Plazi |
|
scientific name |
Mirolabrichthys Herre |
| status |
|
Mirolabrichthys Herre 1927: 413
(masculine; type species Mirolabrichthys tuka Herre & Montalban View in CoL in Herre 1927 by monotypy).
Entonanthias Jordan & Tanaka 1927: 385
(masculine; type species Entonanthias pascalus View in CoL Jordan & Tanaka, 1927 by original designation and monotypy).
Diagnosis. The following synapomorphies support monophyly of the genus:
1. Distinctive dorsal-fin shape. Mirolabrichthys species , particularly adult males, have a distinctive dorsal-fin shape. The fourth to 10 th spines are longest, most of the soft dorsal fin is distinctly elevated above the spinous portion, and the mid-posterior rays are longest, often with prolonged free tips ( Fig. 1 View FIGURE 1 ).
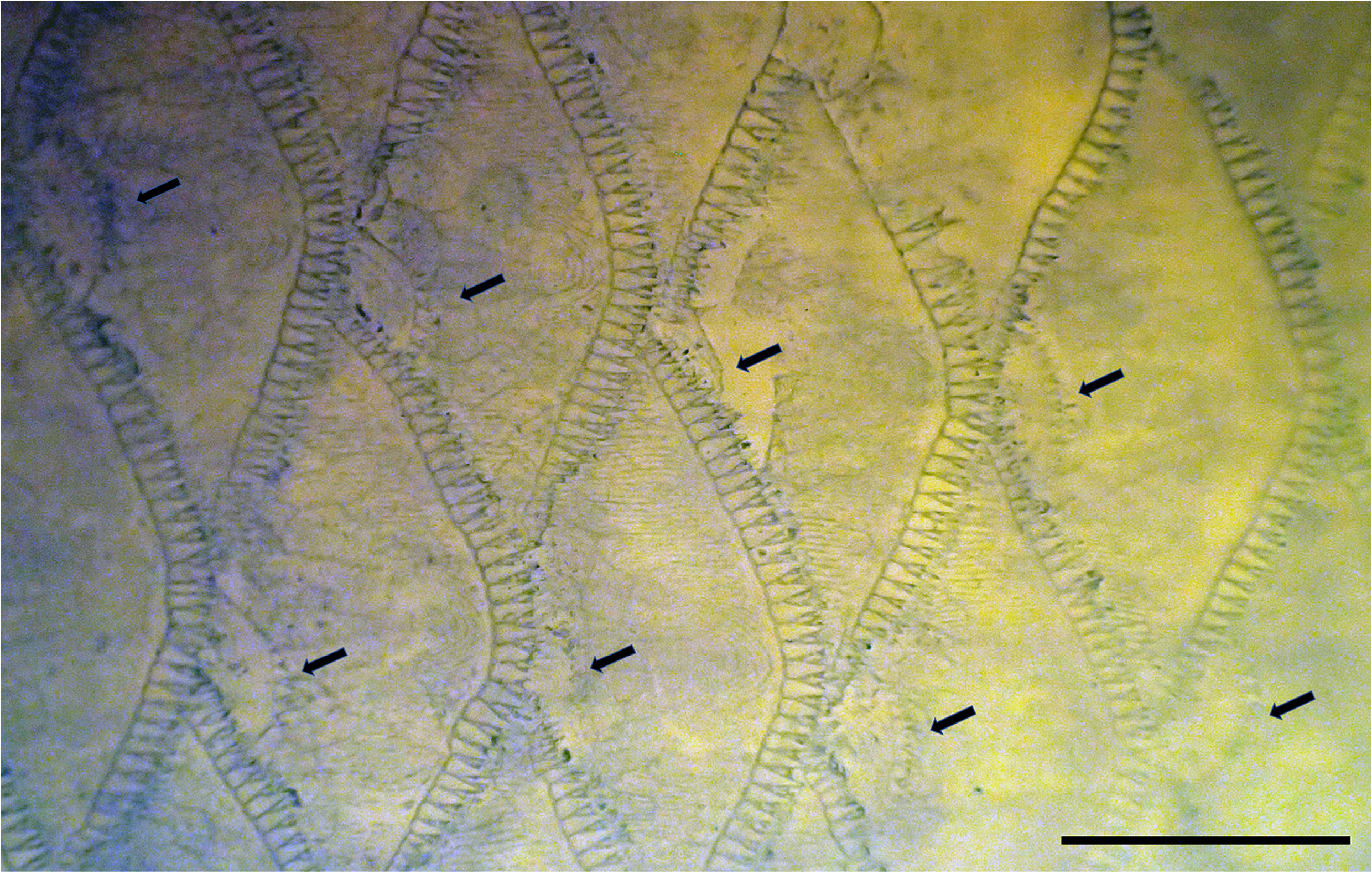
2. Auxiliary scales present on body. Mirolabrichthys species are relatively distinctive in having extensive coverage on the body of auxiliary scales, small scales at the bases of the normal scales ( Fig. 2 View FIGURE 2 ). Auxiliary scales are known from several other anthiadine genera (reviewed by Pogonoski & Gill 2021), although in most these are restricted to the head, nape and anterodorsal part of the body. Among Pseudanthias and near relatives, they are mostly either absent or restricted to the head and anterior body. Only a few species have extensive coverage on the body, including P. thompsoni ( Fowler, 1923) , P. squamipinnis ( Peters, 1855) , P. olivaceus ( Randall & McCosker, 1982) , P. huchtii ( Bleeker, 1857) and P. nobilis ( Franz, 1910) . All but the first species appear to be closely related to each other (e.g., uniquely among anthiadines, males of these species have pigmented blotches on the distal part of their upper pectoral-fin rays), and probably should be classified in the genus Franzia . I consider the extensive coverage of auxiliary scales to be independently derived in Mirolabrichthys .
The presence of auxiliary scales over much of the body, in combination with the following characters distinguishes Mirolabrichthys from other anthiadine genera: pectoral-fin rays 15–19; tubed lateral line scales 45–52; circumpeduncle scales 23–28; vomerine teeth small, in small patch; interopercle and subopercle either smooth or with a few small serrations.
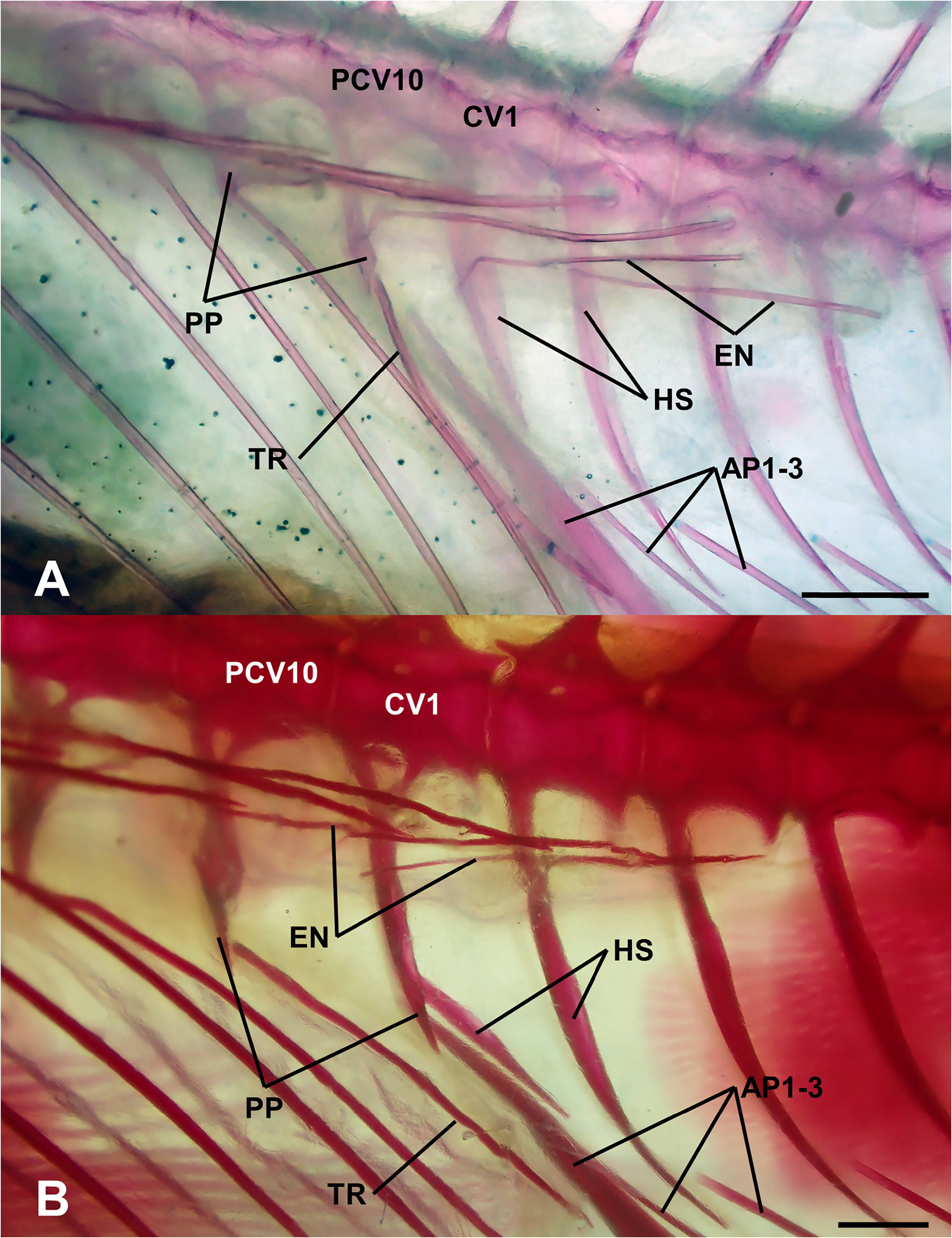
Description. See Tables 1–5 View TABLE 1 View TABLE 2 View TABLE 3 View TABLE 4 for variation among species. Dorsal-fin rays X,15–18; dorsal-fin origin above pectoral-fin base ( Fig. 1 View FIGURE 1 ); primary shaft of first dorsal-fin pterygiophore angled distinctly posterodorsally; ADPF S/S+S/3/1+1/1/1/1/1/1 (= predorsal formula 0/0+0/2/1+1; Fig. 3A View FIGURE 3 ); dorsal-fin pterygiophores in interneural spaces 9–13 1/1/1+1/1+1/1+1, 1/1+1/1+1/1+1/1 or 1/1+1/1+1/1+1/1+1; terminal dorsal-fin pterygiophore in interneural space 17–18; anal-fin rays III,7–9; terminal anal-fin pterygiophore in interhaemal space 5–6; pectoral-fin rays 15– 19, no serrations on rays; pelvic-fin rays I,5; caudal-fin rays 9–11+9+8+9–11; branched caudal-fin rays 7+6; no procurrent spur on upper procurrent ray in lower lobe; penultimate procurrent ray in lower lobe not foreshortened (see Johnson 1975, 1983).
Scales moderately small, tubed scales in lateral line 45–52; circumpeduncle scales 23–28; auxiliary scales extensively present on head and body; lower jaw scaled; basal scaled area on dorsal and anal fins broad; scales with peripheral cteni only ( Roberts 1993).
Greatest body depth 28–37% SL; head length 24–30% SL; orbit diameter 5–7.5% SL; predorsal length 31–37% SL; preanal length 60–65% SL; prepelvic length 31–37% SL; caudal peduncle depth 13.5–16% SL.
Anterior part of upper lip of males hypertrophied ( Fig. 1 View FIGURE 1 ); mouth large, oblique, posterior margin of maxilla reaching to point ranging from vertical through posterior edge of pupil to vertical through posterior edge of eye; mouth terminal, becoming inferior in males; supramaxilla absent; premaxilla with 1 or 2 enlarged canines anterolaterally, a band of small conical teeth about 4 or 5 rows wide at symphysis reducing to 1 or 2 rows on sides of jaw, with outer-row teeth larger, those of posterior half of jaw curved anteriorly or inwards; 1–3 posterior teeth in band nearest symphysis enlarged and caniniform, lying almost flat against roof of mouth; dentary with 1 or 2 laterally curved, enlarged canines at front of jaw, followed by band of small conical teeth about 3 or 4 rows wide reducing to 1 or 2 rows posteriorly, outer-row teeth much larger, those of posterior part of jaw curved anteriorly or inwards; anterior third of dentary with 1–3 enlarged, posteriorly curved canines outside band of teeth; vomer with small patch of tiny teeth; palatine with a narrow band of small conical teeth, 2 or 3 rows wide at widest point; ectopterygoid, endopterygoid, and tongue edentate.
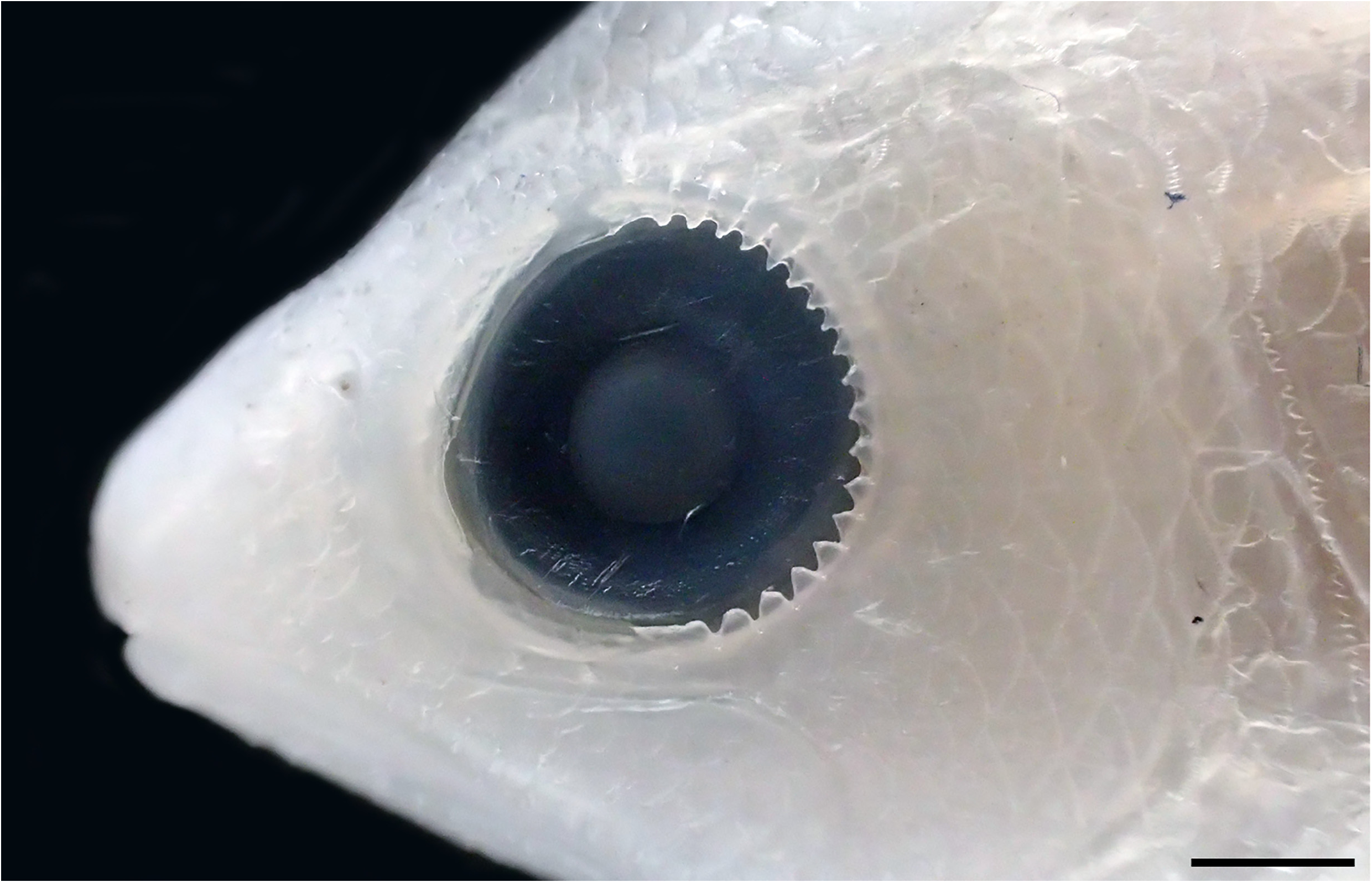
Opercle with three flat spines, uppermost indistinct and covered by scales and skin, lowermost below junction with subopercle, middle spine closer to lowermost spine than to uppermost; preopercle finely serrated at angle and on posterior, vertical edge; interopercle and subopercle either smooth or with a few small serrations; posttemporal smooth or with indistinct serrations or crenulae; posterior rim of orbit with ( Fig. 4 View FIGURE 4 ) or without papillae.
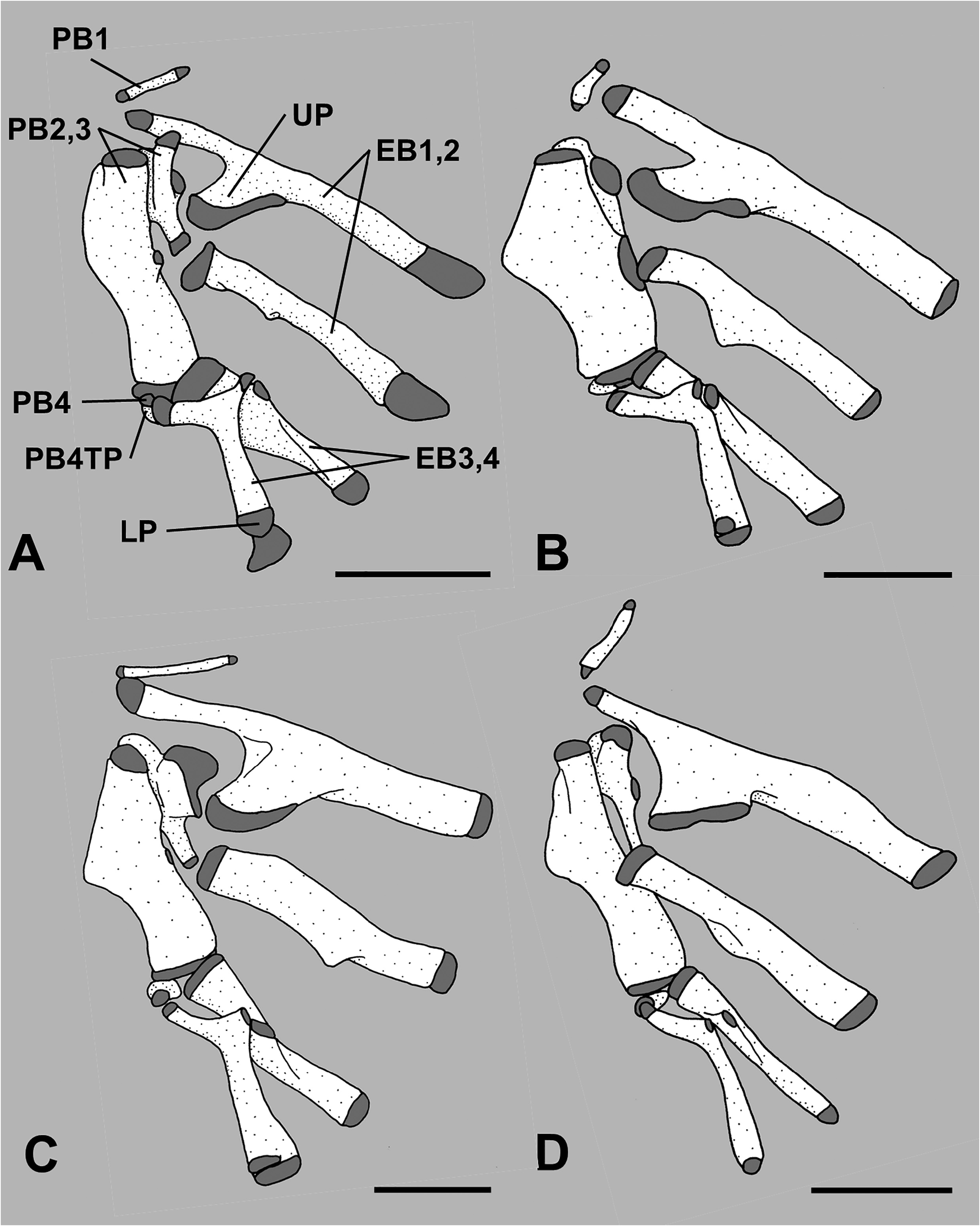
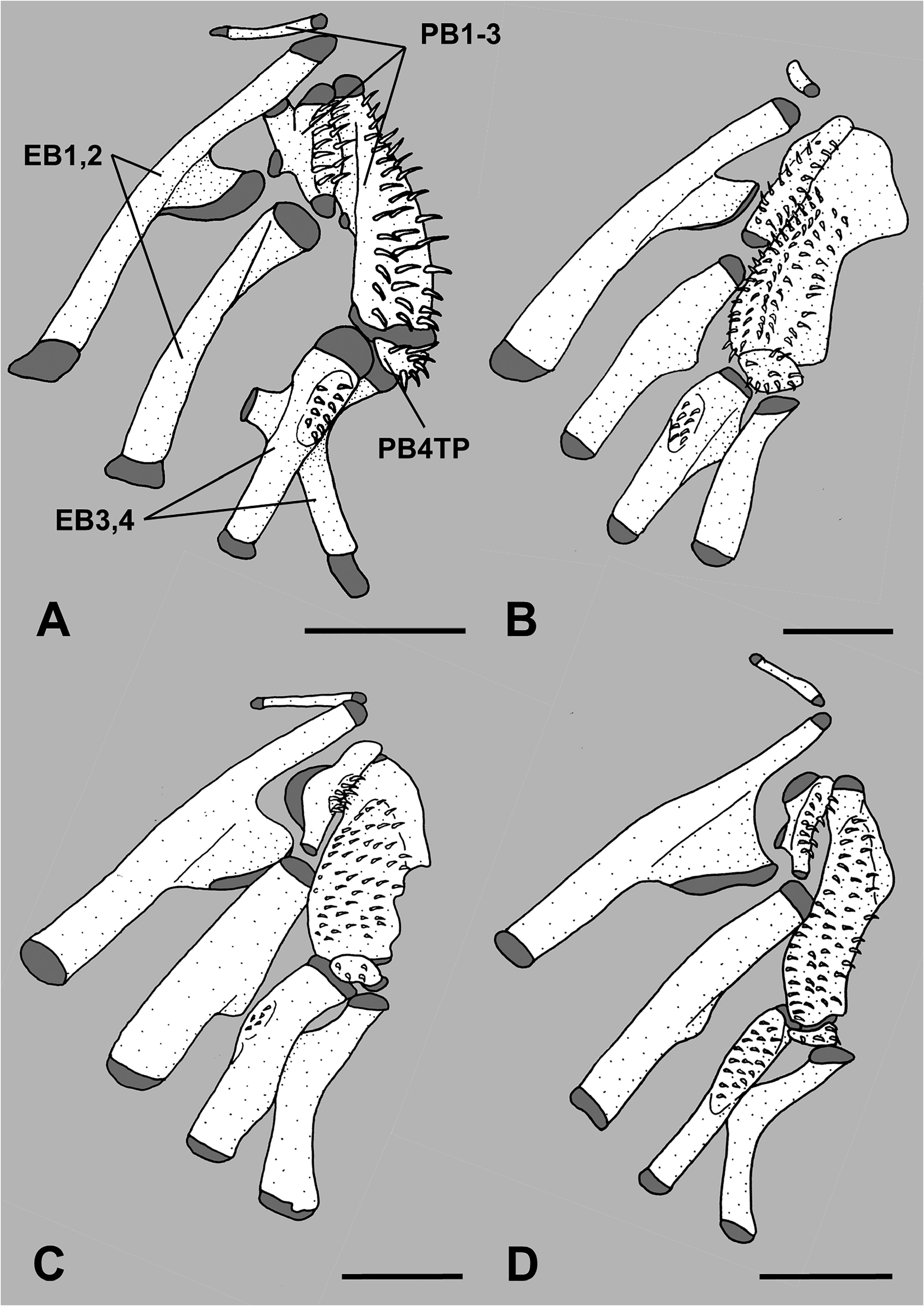
Paired pharyngobranchials (pb) 1 through 4 present, pb4 cartilaginous; tooth plates present on pb2 through pb4, tooth plate on pb4 small and autogenous; paired epibranchials (eb) 1 through 4 present; uncinate process on eb1 fan-shaped, broadly rimmed with cartilage, and directed posterodorsally; no tooth plate on eb2; small toothplate on eb3 ( Figs. 5A View FIGURE 5 , 6A View FIGURE 6 ); paired ceratobranchials (cb) 1 through 5 present; tooth plate on cb5; paired hypobranchials (hb) 1 through 3 present, none with toothplates; median basibranchials (bb) 1 through 4 present, bb4 as cartilage, none with toothplates; gill rakers elongate on anterior/lateral face of first arch, 8–11 on eb1, uppermost at junction with pb1, and 22–27 on cb1 and hb1; shorter club-like rakers or rudiments present on posterior face of eb1, cb1 and hb1, on both anterior and posterior faces of eb2–3, cb2–4 and hb2, and on anterior face of hb3, eb4 and cb5.
Vertebrae 10+15–16; parapophyses present on first caudal vertebra ( Fig. 7B View FIGURE 7 ); epineurals present on vertebrae 1 through 11–13; ribs present on vertebrae 3 through 10; preural 2 (pu2) and pu3 haemal spines autogenous; no radial cartilages in caudal skeleton anterior to pu3 haemal and neural spines; parhypural and hypurals autogenous; welldeveloped hypurapophysis on parhypural; single uroneural; 3 epurals; ventral tip of cleithrum with well-developed posteroventral process.
Composition. Mirolabrichthys tuka Herre & Montalban in Herre, 1927, Entonanthias pascalus Jordan & Tanaka, 1927, Anthias evansi Smith, 1954 .
Remarks. Mirolabrichthys and Entonanthias were described in 1927. Myers (1929) recognised the two genera were synonymous and gave priority to Mirolabrichthys over Entonanthias owing to the former being published in March, whereas the latter was published in June. Myers’s decision has been followed by subsequent authors who have recognised Mirolabrichthys as either a valid genus or a subgenus of Anthias or Pseudanthias . However, Eschmeyer’s Catalog of Fishes (Fricke et al. 2021) provides a date of “27 Jan.” for Entonanthias and “17 Mar.” for Mirolabrichthys , suggesting that Entonanthias is actually the older name. Volume 17 of Annals of the Carnegie Museum includes a statement (p. xvi) that it was published in seven consignments from 9 June 1926 to 27 June 1927, and article 12 (which included the description of Entonanthias ) was in the final consignment. Clearly, the date in Fricke et al. (2021) of “27 Jan.” is a typographical error for “27 Jun.”
All three included species were represented in the phylogenetic analysis of COI sequences by Gill et al. (2021a) and were retrieved as a monophyletic group. Kuiter (2004) recognised two additional species, one for populations of M. pascalus from the Great Barrier Reef and South Pacific, and the other for populations of M. tuka from Rowley Shoals, Western Australia. Further study is needed to address the taxonomic status of these potentially new species.
Material examined. Mirolabrichthys evansi ZRC 62117 (6W,X, 38.8–45.8 mm SL; M. pascalus ASIZP 56149 (1X), AMS I.49578-016 (1W, 72.5 mm SL); M. tuka ACG CS 532 (3 CS, 41–56 mm SL), AMS I.19472-032 (4 CS, 76–84 mm SL), ASIZP 59665 (1X), ZRC 62052 (4W, 29.4–46.0 mm SL).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Mirolabrichthys Herre
| Gill, Anthony C. 2022 |
Mirolabrichthys
| Herre, A. W. C. T. 1927: 413 |
Entonanthias
| Jordan, D. S. & Tanaka, S. 1927: 385 |