Abstract
The crucian carp (Carassius carassius) is a cyprinid fish with its natural distribution in Europe and the western part of Asia. Due to its hardiness and unique ability to survive winter anoxia, it has been translocated to small lakes and ponds, and in Northern Europe since medieval times has been used as a food source. Crucian carp was the only fish in the pond that survived anoxia. Small lakes and ponds with winter anoxia result in dense populations of stunted and slender fish. In lakes with other fish species present, the crucians’ numbers and densities are low and they grow to large sizes. In the presence of piscivores such as pike, crucians are deep bodied. The presence of pike-eating crucians, or the pike odors, induce a change in the body form of crucians. The change in body form makes it more difficult for pikes to swallow crucians and the handling time increases. Closely related invasive Carassius species have become a serious threat to crucian carp populations in Central-Eastern Europe and South-Eastern England through competition for space and food resources and hybridization. The crucian carp’s close relationship to goldfish (Carassius auratus), the most studied species concerning sex pheromones, has made it possible to demonstrate that sex pheromones are also present in a wild Carassius species and in their natural environment. The results indicate that two species use the same sex hormonal pheromone system. The crucian carp has become an important model for laboratory studies of olfaction and taste.
Similar content being viewed by others
Introduction
Ray-finned fishes, Actinopterygii, is a diverse group with more than 30,000 described species and they have colonized and adapted to live in various environments that differ in abiotic variables such as salinity, temperature, oxygen, pH, water current and light and these may vary during the year and day. The majority of ray-finned fishes belong to the group Teleostei including the family Cyprinidae with 2500 species, the family with the highest number of species among vertebrates (Kullander et al. 2012). Cyprinids show great variation in size and appearance and can be found in streams, lakes, ponds and brackish water, but are, with one exception Tribolodon brandtii (https://www.fishbase.se), not present in marine environments. Crucian carp (Carassius carassius (L.)) is one of the species that, due to its unique physiological and behavioral adaptations, has received interest in science. It has also become an important model species for laboratory studies of olfaction. The species is present in small ponds that no other fish survives during winter with ice cover and anoxia and has also evolved features to decrease the risk of predation. In the following text, the species will be presented based on various studies that have been done within the scientific fields of geographic distribution, medieval pond rearing, anoxia tolerance, intra- and inter-species competition, predator-induced morphological and behavior defense, sex pheromones and chemical senses and foraging. This hardy and easy to keep species has also been used in several studies in ecotoxicology and toxicology, but these studies are not mentioned in this review.
Materials and methods
A literature search was done in two databases, ASFA and PubMed, in October 2021. Carassius carassius (mainsubject.EXACT) gave 533 hits in ASFA, year 1915–2021. The titles of all publications were read and 147 of the publications were chosen because the content could be included in the scientific fields of geographic distribution, anoxia tolerance, competition, induced defense, chemical senses, olfaction and foraging. Several hits dealt with Carassius gibelio (gibel carp) and Carassius auratus (goldfish), and the publications were chosen if Carassius carassius was included in the studies or studies on sex pheromones. Studies in toxicology and ecotoxicology and studies on viruses and other pathogens were excluded. To catch studies on, e.g., anoxia tolerance, hybridization, genetics and olfaction that were not included in ASFA, a search was done in PubMed: Carassius carassius [All fields], 1945–2021. Forty-three publications not included in the ASFA search results were found.
Several studies from eastern Asia did, though stated, not use Carassius carassius, as this species is not found east of the catchment area of the Lena River (Libosvársky 1962; Lelek 1987; Szczerbowski and Szczerbowski 2002; Gu et al. 2021). In eastern Asia, there are at least four other species of Carassius (e.g., Takada et al. 2010; Yamamoto et al. 2010; Gu et al. 2021). The common name for Carassius carassius is crucian carp (https://www.cabi.org/isc/datasheet/90564; https://www.Fishbase.se; Kottelat and Freyhof 2007; Kullander et al. 2012), but has also been used for gibel carp and goldfish. The use of the common name crucian carp should be restricted to Carassius carassius. In addition to the literature survey, we scanned reference lists of relevant publications. The section about early crucian carp pond culture is based on old literature and on our own studies (e.g., Bonow et al. 2016).
Results and discussions
Geographic origin in Europe. A phylogeographic study has shown that there are two lineages of crucian carp (Carassius carassius will in the following text be mentioned as crucian/-s) in Europe, one found throughout Northern and Central-Eastern European drainages and a second confined to the Danubian catchment (Jeffries et al. 2016). These lineages have been isolated for about two million years. The development of two distinct lineages is probably due to crucians’ preference for lentic waters (small lakes, ponds) and their low propensity for dispersal. The colonization of Northern and Eastern Europe was probably from the Ponto-Caspian region. The southern lineage is confined to the Danubian catchment. The colonization of the Baltic Sea from mainland Europe after the last ice age may have coincided with the freshwater Lake Ancylus stage from ca. 10,600 to 7500 years ago (Jeffries et al. 2016; see also Lõugas 1999). There is strong genetic similarity between populations from the Åland Islands, southern Finland and central Sweden. Data also suggest routes through Denmark to southern Sweden and an eastern route from Poland. Paleolithic and Holocene (from 11,700 BP) bone remains from crucians and other eurythermic cyprinids have been found in the lakeland of the Polish Lowland (Makowiecki 1999, 2000; Zabilska-Kunek et al. 2016). Fossil remnants from the Pliocene have shown that crucians were already present in southern Ukraine at least during the Pliocene, 2.6–5.3 MY BP (Kovalchuk 2014).
Previous results have shown that crucians from southern Sweden are distinct from those in central Sweden (Janson et al. 2015). The populations in central and Northern Finland probably have an eastern origin (Jeffries et al. 2016). Genetic studies suggest that crucians are not native to England, but were introduced during medieval times followed by a more recent fifteenth-century introduction (Jeffries et al. 2017). Crucians have also been introduced in lakes in Southern Europe (e.g., Volta et al. 2018). They have also been introduced in large lakes in the UK, for instance in the English Lake District and in Loch Lomond and Loch Rannoch in Scotland (Fraser and Adams 1997; Winfield and Durie 2004; Winfield et al. 2011). It has been suggested that crucians are reproducing (e.g., Adams 1994). A recent study did not, however, catch any crucians (Winfield et al. 2011). In Norway, crucians have a natural south-eastern distribution, entering this area from southern Sweden during the time of Ancylus Lake (Øksnevad et al. 1995; Hufthammer and Moe 2016). The presence of crucians in coastal waters in western and southern Norway, especially in the Middle Ages trade centers Bergen and Trondheim, are probably the result of early artificial introductions (Øksnevad et al. 1995; Hufthammer and Moe 2016). Medieval monasteries were located in these two regions. The contemporary distribution of crucians in the Baltic Sea region has been influenced by human translocations to ponds and used as a food source in monasteries, and later in vicarages and mansions (Bonow et al. 2016). The most northern location with introduced crucians is located in Tromsø, Northern Norway (Jeffries et al. 2016). One hundred crucians were introduced into the Lake Prestvannet in 1883 (Karl Oystein Gjelland, NINA, Tromsø, personal comm.). There is only crucian carp and three-spine stickleback (Gasterosteus aculeatus) in the lake. Great numbers of dead sticklebacks in later winter and spring indicate hypoxia/anoxia conditions during winters. In late July 2013, 18 crucians were trapped in Lake Prestvannet, all within 8–13 years old (47–293 g) (age determined with scales) and in spawning conditions (Olsén and Gjelland, unpubl. results). Crucians spawn when the water temperature during spring and early summer increases to at least 18°C (Aho and Holopainen 2000; Olsén et al. 2006). They spawn twice with a two-month delay. As the temperature is crucial, spawning may not occur every year in populations introduced to ponds and small lakes in northern Scandinavia. They also need water plants where females, during intense courting by a batch of males, attach the eggs (Aho and Holopainen 2000; Olsén et al. 2006; Kullander et al. 2012). Crucians’ hardiness and ability to survive winter anoxia and the possibility to be transported in moist moss made the species popular to stock in ponds and use as a food source (Ekström 1831; Bonow and Svanberg 2011; Svanberg et al. 2012). It was always possible to get fresh fish.
The species have also proven totally resistant to the trematode parasite eye fluke (Diplostomum spathaceum), which is exceptional and probably related to crucians’ unique physiology (Karvonen et al. 2005). The trematode is one of the most prevalent parasites in freshwater fish. The presence of other monogenean host-specific parasites in crucians are probably regulated by the anoxia conditions present in ponds (Bagge et al. 2004).
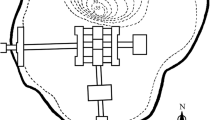
Crucian carp in pond culture. Crucians have been used in pond breeding to get fresh fish since late medieval time until around 1900. Due to its hardiness and unique anoxia tolerance (see the following chapter Anoxia tolerance), it was the only fish that could be stored in small ponds year around. It was always possible to get fresh fish. The present knowledge about crucian carp ponds is based on pond remnants and geometrical cadastral maps are an essential source category (Fig. 1). Such maps have been shown to be a useful source for studying garden culture, orchards, mills and other economic activities on farms and manors in former times (Nilsson 2010). In Sweden, only few archeozoological remains from medieval or early modern ponds have been analyzed so far (Jonsson 1984; cf. Nordeide and Hufthammer 2009). Judging from the survey maps, crucian carp ponds were once common in southern and central Sweden, mainly in the eastern parts of the country. The same situation was found in Swedish Finland (Bonow and Svanberg 2016).
An example of a cadastral map, Skabersjö Castle, Scania, Sweden (Lat. 55.54, Long. 13.15). The moat, ponds and barns from 1768 are still present in 2011. All ponds contained only crucian carp. The pond C in the lower map had a dense population of crucians (see Fig. 4). Swedish Land Survey Board archive, LMS, L171-10:2, Skabersjö 1768.
Crucian carp ponds can still be found at castles, manors, vicarages and village commons, but most have now disappeared (Bonow and Svanberg 2012, 2016, 2018). One of the earliest written sources are from The Charles Chronicle (Karlskrönikan), which claims that the first time a fish pond was founded in the country was at the Vyborg Castle, a Swedish built medieval fortress on the Carelian isthmus, in 1446. It was the military governor Karl Knutsson (Charles, later king of Sweden) that commissioned kroppa damber (‘crucian carp ponds’) to be constructed (Klemming 1866: 245). One other early source is from northern Island Gotland where a rwde dam (‘crucian carp pond’) was established around 1485 by Privy Council Ivar Axelsson Tott (Fabricius 1904: 278; Melfors 1991: 173). Several ponds with crucians were present and supplied the fortress with fresh fish (Rasmussen 1959; Svanberg et al. 2012). The Island Gotland was an important trading port of the Hanseatic League, a commercial federation that dominated in Northern Europe from the 13th to 17th centuries. Crucians and other cyprinids could have been an important food source.
There is a common, but uncorroborated opinion that aquaculture was introduced in Scandinavia with the monastic culture, but very few reliable sources about monastic fish ponds exist from Sweden (Bonow and Svanberg 2016). Archeological finds from monastic fish ponds are also few, so hardly any archeozoological material has been studied (Lepiksaar 1969). Certainly, some traces of fish ponds are known from Swedish monasteries. From Vadstena Abbey, which belonged to the Bridgettine Order, rudho damber ‘crucian carp ponds’ obviously used for raising fish are mentioned in 1470 and 1517 (Karling 1928: 165; Bernström 1969: 441). Similarly, there is still a partially retained pond at the Franciscan convent (Ordo Fratrum Minorum) in Söderköping. Other Franciscan convents with fish ponds are present in the city of Linköping (Tagesson 2000: 222). The fish pond excavated at Nylödöse might originally have been part of the Franciscan convent (1473–ca. 1520), or, as has been suggested by historian Rune Ekre, from an earlier abbey belonging to the Dominicans (Ekre 2007). Some insight into the late medieval monastic fish ponds in Sweden is given by Petrus Magni (Peder Månsson) in his manuscript Bondakonst, written around 1520. He was ordained a monk brother in Vadstena Monastery of the Bridgettine Order. In his manuscript from 1520, Petrus dedicates a whole chapter to pond fish culture (Svanberg and Cios 2014). Another interesting document is the instruction provided by Bishop Hans Brask in Linköping to the state overseer at the Bishop’s House, where he was ordered to perform a special supervision of rudadammom (the crucian carp pond), from where fish were sold on the market (Bonow and Svanberg 2016). The pond cultivation in castle and monastery grounds that had been established in Sweden during the late medieval times lived on as a production form after the Reformation. Scattered data in the sources shows that several castles had crucian carp ponds in the sixteenth century (Nordström and Dahlander 1908: 31; Bonow and Svanberg 2012: 134, 140). Fish production in ponds seems to have been common places in the grounds of Swedish castles during the sixteenth and seventeenth century (Nilsson 1939; Bonow and Svanberg 2016).
In Scania, we can observe an interest in large-scale fish farming in the eighteenth century on some of the larger estates. In Skånska resa (1751), Carl Linnaeus described the estate of Marsvinsholm in Scania where there were 99 ponds, including one on a roof, containing common carp and crucian carp (Svanberg et al. 2012). What significance crucian carp ponds had for the supply of estate households has not been investigated, but these systems of ponds were obviously built in order to sell fish (Bonow and Svanberg 2016).
Anoxia tolerance. Crucian is a eurythermal fish that survives within a wide temperature interval and can survive down to 0°C (Lenkiewicz 1964; Horoszewicz 1973; Holopainen et al. 1997a). The species is heat tolerant (Horoszewicz 1973; Laurila et al. 1987). The optimal temperatures for egg survival and development are between 21 and 28°C (Laurila et al. 1987) and the fastest growth rate in larvae and juveniles was at 28–30°C. Sikorska et al. (2018) found that the highest survival and growth rates in larvae and juveniles were between 25 and 28 °C. Behavior disturbances and death have been observed at 35–36 °C and 38 °C, respectively, after being kept at 27 °C (acclimation temperature) (Horoszewicz 1973). Crucian is one of few vertebrates that survives anoxia (Vornanen et al. 2009). This was first studied by Blažka (1958), who observed that crucians can survive up to 5.5 months of anoxia in natural ponds. This was later confirmed by Hyvärinen and Holopainen (1983). Piironen and Holopainen (1986) studied the seasonality of anoxia tolerance in the laboratory and found long survival times during the winter, 4.5 months at +3 °C, when the glycogen content in the liver was high. In spring and summer, crucians survive only one day in anoxia (Fig. 2). A key factor to survive anoxia in natural populations is the access to food (mainly cladocerans and chironomids) during summer, which is problematic in dense populations with high competition that result in reduced food resources (Paszkowski et al. 1990). This results in low glycogen reserves in the liver and high winter mortality, especially in larger individuals (Tonn et al. 1994). The closely related goldfish also survive anoxia, but only from several days to two weeks (Walker and Johansen 1977). Crucians have high hemoglobin concentration in the blood, which has high affinity to oxygen (Belchenko and Kel 1991; Kamshilov and Kamshilov 2019) that is important during hypoxia. Further, the gill respiratory surface in crucians increases several times within a few days under hypoxic conditions. There are reversible morphological changes that increase the area of secondary lamellae in contact with the water and increase oxygen uptake (Sollid et al. 2003). Mitochondria density in muscles is higher in cold-acclimated (2 °C) crucians compared to crucians acclimated to higher temperatures (28 °C) (Johnston and Maitland 1980).
Seasonality of oxygen content and temperature in the pond Hermanninlampi, Finland. Glycogen reserves (sum of liver and white muscle) and lipid metabolism were found in crucians caught in the pond. The experiments in the laboratory demonstrated that crucians survived a longer time during anoxia in winter, but only a short time in summer. The anoxia tolerance was high in winter, stated as high LT50, but low in summer, low LT50. Figure from Piironen and Holopainen (1986). Reproduced by the permission of the authors and Annales Zoologici Fennici
Ice cover during winter reduces the oxygen content in ponds and small lakes, and crucians are often the only species and no piscivores are present (Öhman et al. 2006). The key to anoxia tolerance in crucians is ethanol that is produced from glycogen instead of lactic acid (Johnston and Bernard 1983; Holopainen and Hyvärinen 1985; Holopainen et al. 1986). Ethanol, in contrast to lactic acid, is released through the gills. Great amounts of glycogen are stored during the summer before the temperature decreases and the pond is covered by ice. The glycogen stores in the liver are obligatory for survival under anoxia conditions. Small crucians have much higher liver weights than large fish (Vornanen et al. 2011). During July after spawning in early summer, the crucians increase their liver weight during foraging to over 10% of the body weight and the liver’s glycogen content can be 35% (Hyvärinen et al. 1985; Vornanen et al. 2009). White muscle glycogen stores have some importance in large fish (Johnston 1975; Vornanen et al. 2011). The glycogen stores exceed the demands during the winter and there is still glycogen present in the liver in spring. There are also high glycogen contents during the winter in the brain and cardiac muscle to be able to handle anaerobic glycolysis. Crucians are still physically active, although at a reduced level and hearing and vision are decreased during anoxia to reduce energy use (Nilsson et al. 1993; Johansson et al. 1997). Glycogen content in the heart and brain shows seasonal cycling, reflecting the oxygen decrease and increase in water, with the highest glycogen content in midwinter and lowest in summer (Vornanen and Paajanen 2004, 2006). Crucians have also been present for at least two million years in regions with no winter ice cover (Jeffries et al. 2016) and it would be interesting to know if they are as good to survive anoxia as the populations in Northern Europe. Crucians overwinter in periodic torpor in the bottom mud during anoxia and they may be able to survive bottom freezing (Nilsson 1855; Holopainen et al. 1997a; Kullander et al. 2012). As far as we know, no experiment has been done to show that crucian carp can survive freezing. It may be possible that crucians during torpor in bottom sediments of small ponds survive winter anoxia by the freeze point lowering effects of ethanol. There are some other vertebrates such as at least four amphibians and some reptiles that tolerate freezing (Willmer et al. 2005). Glycerol, glucose and amino acids are used to decrease the freezing point. In the North American wood-frog Rana sylvatica, massive amounts of glycogen are built up in the liver before hibernation, and synthesis of cryoprotective glucose is triggered by ice on the skin. High concentrations of glucose are present in the liver, heart and brain (Willmer et al. 2005). In Antarctic fish such as Pagothenia borchgrevenski, the freezing point is reduced by increased sodium chloride levels in the blood and production of antifreeze glycoproteins in the liver that are released into the blood. Antifreeze peptides/proteins are also produced by Arctic fish, but in contrast to Antarctic fish the production is seasonal and controlled by the photoperiod (Willmer et al. 2005).
Competition with closely related species. In addition to crucians being sensitive to competition, they hybridize with closely related species such as gibel carp (Carassius gibelio) and goldfish (Carassius auratus). Most of the studies on the effect of gibel carp on populations of crucians have been done in Eastern Europe. In these parts of Europe, crucians have had some commercial value and gibel carp have been cultivated at least since the 1960s (Mickiewics and Wołos 2012; Kuljanishvili et al. 2021). Gibel carp (hereafter mentioned as gibel) has a more eastern natural distribution than crucians and is an invasive species that in some populations is composed of only triploid females that use sperms from crucian males and males of other cyprinids to induce egg cell division resulting in clones of offspring (gynogenetic reproduction) (e.g., Vetemaa et al. 2005). Paternal genes can in rare cases be transmitted to the offspring (Tóth et al. 2005). Gibel have been introduced to Eastern Europe from Asia (e.g., Rylková et al. 2013). It has become very common in the south-eastern and eastern parts of the Baltic Sea coast (Vetemaa et al. 2005). In addition to sexual parasitism, crucians and diploid gibel hybridize and compete for resources (e.g., Wouters et al. 2012; Busst and Britton 2015). In locations where gibel have been introduced by accident or by intention from hatcheries, the species has become the dominant species and replaced crucians by competition and hybridization (e.g., Papoušek et al. 2008; Tarkan et al. 2016; Mezhzherin et al. 2019; Kuljanishvili et al. 2021). In some parts, hybrids are more common than pure crucians (Mezhzherin et al. 2019). Analyses of mtDNA haplotypes have shown that hybrids in some locations are offspring between gibel females and crucian males (Papoušek et al. 2008; Wouters et al. 2012). In the Czech Republic, a drastic decline in previously healthy crucian populations and in some cases extirpation of local crucian population have followed the spread of gibel (e.g., Lusková et al. 2010) and habitat degradation (Lelek 1987; Tarkan et al. 2016). In other parts of Europe, competition with gibel or goldfish (Carassius auratus) has also been recognized as a significant factor in the decline of crucian populations and other native species (e.g., Lelek 1987; Wheeler 2000; Copp et al. 2010; Lusková et al. 2010). Pond experiments have shown that gibels have strong negative effects on crucians (Demeny et al. 2009). The mechanisms behind the negative effects are not known, but it has been shown that juvenile crucians kept together with gibel juveniles decrease in numbers and show less growth rate compared to groups with no gibels present.
There are attempts to artificially reproduce and reintroduce crucians (Myszkowski et al. 2002; Żarski et al. 2011, 2014; Targońska et al. 2012; Łączyńska et al. 2016; Sikorska et al. 2018; Kasprzak et al. 2019; Sayer et al. 2020). In certain areas in Sweden, crucians are still common in small lakes and ponds with no other fish present. They are also present in Baltic Sea coastal waters, but their presence is not fully known. Large fish are occasionally caught. It is possible that the crucian populations are already impacted by the presence of gibels. Further, there is also a problem in distinguishing crucians from gibels and their hybrids. Large hybrids have been caught in three locations on the Swedish Baltic Sea coast and the D-loop mitochondrial sequences showed that they represented linages of gibelio from China, Japan and Russia (Wouters et al. 2012). It is not known how and when gibelio arrived in these locations. There is evidence from Finland, where gibels are present in high numbers in coastal waters, that they have started to spread into ponds connected to the sea and recently even in isolated ponds with crucians (Dr. Lauri Urho, Natural Resource Institute Finland, personal comm.), therefore increasing the future threat to crucians. Gibel carp occurrence has been documented in the Turku (Åbo) area on the southwest coast of Finland (Finnish Biodiversity Information Facility; GBIF Finland) not far from Åland Island. When the species is present in the Åland Archipelago, it will probably not take long before gibels reach the Swedish coast. As described above, the morphological identification of hybrids is difficult and therefore the true extent of this problem can only be revealed by molecular methods (e.g., Hänfling et al. 2005; Wouters et al. 2012; Bernos et al. 2020).
Predators induce changes in body form and behavior. In Northern Europe, two forms of crucians can be found, one stunted form living as the only species in small lakes and ponds in high densities and strong intraspecific competition and the second form living in lakes in low densities with piscivore species present. The pond form is small and slender, while the lake form is deep bodied and grows up to 4–5 kg (Paszkowski et al. 1989; Holopainen et al. 1997a) (Figs. 3, 4, 5). Previously, the two forms were designated as two different species or subspecies (e.g., Ekström 1831; Szczerbowski and Szczerbowski 2002). The opinion that the forms were different species was disputed by Ekström (1839), who demonstrated that the body forms were influenced by the environment. The difference in the two body forms is also related to the presence or absence of piscivores (Holopainen and Pitkänen 1985; Piironen and Holopainen 1988; Tonn et al. 1992; Brönmark et al. 1995; Poléo et al. 1995; Holopainen et al. 1997a) (Fig. 6). The two forms are not genetically determined, but reflect phenotypic plasticity. Shallow-bodies individuals can be changed to high-bodied crucians either during the presence of predatory fish or when crucians are introduced to productive lakes without fish (Holopainen et al. 1997b). Vøllestad et al. (2004) found in the laboratory a small, but significant difference in body depth between crucians exposed to pike-scented water for 14 weeks and controls not exposed to pike water. Body depth increased in both groups. In an experiment where a small pond was divided in two halves, one half with pike (Esox lucius) and crucians and the other half with only crucians, the crucians with the pike developed higher bodies (Brönmark and Miner 1992). The results indicate that the presence of pike is important to get a phenotypic change and there should be close interactions, as no body change was induced in the pike free part (Fig. 7). The experiment was followed by a study in the laboratory where crucians and pike were placed in an aquarium divided into two compartments (Fig. 8). Pike is an efficient predator of crucians (Greenberg et al. 1995; Lappalainen et al. 2013). These initial experiments showed that the phenotypic change was caused by the presence of predators (Brönmark and Miner 1992; Brönmark and Pettersson 1994; Holopainen et al. 1997b). Further, Brönmark and Pettersson (1994) demonstrated that the phenotypic change to a deep body was induced by chemical cues from pike or perch (Perca fluviatilis) that have been eating crucians, but odors from perch eating chironomids or crucian alarm chemicals from skin resulted in no change. Skin extracts from conspecifics induce olfactory-mediated fright responses in crucians (e.g., Hamdani et al. 2000). Stabell and Lwin (1997) showed that odors from pike-eating Arctic charr (Salvelinus alpinus) resulted in, contrast to a diet of crucians, no morphological changes in crucians. Later, Stabell et al. (2010) confirmed the results and demonstrated that chemical alarm signals were not responsible for the phenotypic change (Stabell et al. 2010). The authors suggested that the active odors were from tissue metabolized by intestine bacteria in predators and released into the water.
One female crucian carp (1,820 g) out of 32 caught (range 507–1820 g, median 928 g, 25% percentile 812 g, 75% percentile 1,075 g) on the Baltic Sea coast (Lat. 60.26, Long. 18.37) during fishing with hoop net in June 2011 and June 2012. All crucians were deep bodied and had body scars indicating pike attacks. The main goal of fishing was to reduce the dense population of bream (Abramis brama) and crucians were occasionally caught. Tissue samples from crucians sampled were included in Jeffries et al. (2016)
Height index [(height/total length) × 100] of crucians caught on the Baltic Sea coast (Fig. 3) (solid circle) and crucians from two pond populations. Open circles Lake Prestvannet, Tromsø and squares Skabersjö Castle, Scania (one specimen shown in Fig. 4). Three-spine stickleback is present in Lake Prestvannet. There is a significant negative correlation between TL and HI among the Baltic Sea crucians
Height index of crucians from a multi-species lake (A) and from a pond with only crucians (B). Figure from Holopainen et al. (1997a). Reproduced with the permission of the authors and Annales Zoologici Fennici
The relation between body length (L) and body depth (D) of crucian carp in the presence and absence of pike in two ponds. The ponds were divided into two equal sized sections with a plastic curtain tightly attached to the bottom sediments. A number of pikes were introduced to one of the sections. The regressions were significantly different between the treatments in both ponds. Figure from Brönmark and Miner (1992). Reproduced with the permission of the authors and Science
The body depth–length ratio of crucian carp at low- and high-food levels and in the presence of pike. Crucians and pike were placed in an aquarium divided into two compartments of equal size by a plastic screen partition. Crucians could see, smell and hear the pike. Pike were fed three times a week with one crucian. Treatments differed significantly, analysis of variance P < 0.001; Tukey’s test (single asterisk P < 0.05, triple asterisk P < 0.001). Figure from Brönmark and Miner (1992). Reproduced with the permission of the authors and Science
Crucians exposed to predator odors showed also reduced locomotor activity (Holopainen et al. 1997b; Pettersson et al. 2000; Vøllestad et al. 2004). An acute contact with pike-scented water also increased heart and respiration rates in crucians compared with controls with no contact with pike (Holopainen et al. 1997b). The fish were caught in a pond with only crucians present. After 115 days with pike, the heart and respiration rates and oxygen consumption decreased compared to controls. The swimming activity was also lower. The authors suggested that the reduced swimming activity and energy consumption resulted in the higher growth rate observed in crucians exposed to pike (Holopainen et al. 1997b). Both starved pike and those recently eating crucians and roach had effects on crucians’ activity (Pettersson et al. 2000). Crucians from populations coexisting with pike responded less to pike-scented water compared to crucians without prior experience. Reduced locomotor activity was also shown during exposure to water scented by pike-eating crucians for a longer time (Pettersson et al. 2001). Swimming speed was reduced in crucians eating live zooplankton, but there was no effect in crucians eating dead chironomids, when pike-scented water was added (Andersson et al. 2006). Crucians eating zooplankton had, before exposure to pike odor, much higher swimming activity compared to fish-eating chironomids. The body shape was affected by both diet and pike scent exposure. Crucians exposed to pike had higher body, and the same was observed in crucians eating chironomids. Odors from perch-eating crucians also decreased activity in crucians. Perch-eating chironomids had no effect. Crucians exposed to pike showed, in contrast to controls, no activity peaks during early and late night. Further, the field closure experiment showed that the presence of piscivores increased crucians’ use of vegetation and they moved less (Greenberg et al. 1995). In an earlier study, both fed and hungry crucians spent less time in an open space with tubifex worms when a pike was present in an adjacent compartment of the aquarium (Pettersson and Brönmark 1993). The crucians could both see and smell the pike. The importance of the vegetation zone for young crucians’ escape from piscivores and survival has also been observed in a lake experiment before they attain a size refuge from predation (Tonn et al. 1992).
As mentioned before, the heart beat and respiration rates were reduced in contact with pike odors (Holopainen et al. 1997b). The authors suggested that these changes save energy to facilitate the growth in body height. Another possibility may be that initially these changes in crucians are directly caused by the presence of predators’ odors to decrease the predators’ ability to locate the prey. Freezing is in general a common behavior in stressful situations among animals. Ventilation rates can also change by exposure to relevant odors (e.g., Murphy et al. 2001). The phenotypic change due to the presence of predators was suggested to increase the handling time and increase the risk to be exposed to even larger predatory fish (Nilsson et al. 1995). Auditory cues in connection to prey handling are learned and can increase the risk of predation (e.g., Wisenden et al. 2008). Crucians are sensitive to predation (Tonn et al. 1989) and phenotypic change should be an important adaptation. Further, high-bodied crucians have a higher ratio between the muscle mass and body weight than slender fish (Domenici et al. 2008). They also show superior acceleration and higher turning rates than shallow-bodied crucians, that is deep-bodied have enhanced escape locomotor performance. Further, the rib bones in high-bodied large crucians are large and strong and like a carapace which should give additional protection against pike attacks (Olsén, personal observations).
The physiological mechanism behind the odor-induced phenotypic change is not known, but confrontations with predators are stressful situations and the HPI axis (hypothalamus–pituitary–interrenal axis) and stress hormones can be involved. Fright responses to skin extracts are followed by increased cortisol levels in crucians, and by blocking the HPI axis the behavior response was suppressed (Lastein et al. 2008b). In a recent study, cortisol treatment of crucians made the fish darker, but the body height was not changed (Vinterstare et al. 2020). Goldfish exposed to a predator, bluegills (Lepomis macrochirus), in the same tank had increased blood plasma levels of cortisol (Kagawa and Mugiya 2000). Enhanced cortisol levels were also observed when the predator was separated from goldfish with a net partition or a transparent wall. Only water from the predator showed no stress response. Previous studies with salmonid fish have shown that social interactions between dominant and subordinates result in stress and that subordinates get darker skin (O’Connor et al. 1999; Höglund et al. 2000). The subordinates had higher activity of the serotonergic system which is connected to stress (Höglund et al. 2000; Backström and Winberg 2017). A change in serotonergic activity may also be the case in crucians interacting with predator fish. Crucians were in a recent study exposed to the selective serotonin reuptake inhibitor (SSRI) fluoxetine in the ambient water with or without the presence of pike (Vinterstare et al. 2021). Exposure to a high dose for two weeks made the fish shyer (took longer time to leave a refuge box) and the change was more pronounced with one pike present. Other studies have shown that the SSRI citalopram increases boldness in three-spine stickleback (Gasterosteus aculeatus) (Kellner et al. 2016), Endler’s guppy (Poecilia wingei) (Olsén et al. 2014) and zebrafish (Danio rerio) (Nielsen et al. 2018). The body shape was changed, with higher body depth, in the presence of pike with or without SSRI. Surprisingly also high doses of SSRI without pike present changed the body form, but differently compared to the ordinary response to pike. Increase in body depth during exposure to water conspecific skin extracts has also been observed in goldfish (Carassius auratus) (Chivers et al. 2008). The chemical identity of the compound/-s acting as signals to induce phenotypic changes in Carassius are still to be identified. The knowledge of the compounds should make it easier to reveal the mechanisms. It has been shown that neurons in certain parts of the olfactory bulbs of crucians are stimulated by conspecific skin extracts that induce behavior alarm reactions (Hamdani et al. 2000; Hamdani and Døving 2002, 2003). These neurons can, by electrophysiological methods, be useful to identify the odor molecules active to induce fright responses. Based on single unit recordings in crucian carp olfactory bulbs, it has been suggested that not only alarm substances that add information about species identity, but also other substances are involved (Lastein et al. 2008a). It will be more problematic to identify the compounds that induce phenotypic changes, as their effects are not acute.
Crucian sex pheromones resemble goldfish pheromones. Goldfish is one of the most, probably the most, studied fish species concerning sex pheromones (e.g., Stacey 2015). The pheromones are hormones or hormone metabolites released into the water in connection to key reproductive processes (Scott and Ellis 2007). To confirm if the same type of pheromone system is also present in the closely related wild crucians, a number of experiments were performed both in the laboratory and in the field (Bjerselius and Olsén 1993; Bjerselius et al. 1995a, b; Olsén et al. 2006; Stacey et al. 2012). Initially, the olfactory sensitivity in crucians to the sex hormones 4-pregnen-17,20β-dihydroxy-4-pregnen-3-one (17,20β-P) and its glucuronated metabolite 17,20β-P-glucuronide, and prostaglandin F2α (PGF2α), all acting as hormonal pheromones in goldfish (e.g., Sorensen et al. 1988; Stacey et al. 1989), were studied with electro-olfactogram (EOG) (Bjerselius and Olsén 1993). 17,20β-P is important during the final maturation of eggs and sperms in female and male goldfish, respectively. PGF2α is important for ovulation. Both hormones are released into the ambient water and can act as a preovulatory priming pheromone and a postovulatory releaser pheromone, respectively. Priming pheromones have effects on the physiology of the fish that detects them, and releaser pheromones have effects on the behavior. Mature goldfish males exposed to 17,20β-P in the ambient water had increased volumes of strippable milt. PGF2α that is released in connection to ovulation induces courtship and spawning behaviors (Stacey and Sorensen 2006; Stacey 2015). Injection of PGF2α into males or nonmature goldfish induces female spawning behavior and they are courted by mature males (Shinohara and Kobayashi 2020). After PGF2α injection, nonmature crucians show female spawning behavior and are courted by mature goldfish males (Olsén, personal observations).
Goldfish have become an important model concerning cyprinid hormonal pheromones and it is clear that there are complex interactions with hormones and behavior between the sexes that synchronize spawning (Stacey 2015). The goldfish has, however, been reared in captivity for generations and there is a possibility that they have gained the ability to respond to sex hormones as pheromones. To investigate if the hormonal pheromone system in goldfish is also present in closely related wild crucians, several studies were conducted both in the laboratory and in ponds. The EOG measurements revealed that crucians were sensitive to 17,20β-P, but not to 17,20β-P-glucuronide. They detected 17,20β-P down to 10-11 M, which was in our study 10 times lower concentration than that in goldfish (Bjerselius and Olsén 1993). Spermiating and immature crucians had about the same sensitivity to 17,20β-P, i.e., they had functional receptors independent of the state of maturation. It was different with PGF2α—immature crucians had low olfactory response to the hormone compared to spermiated crucians and goldfish. The importance of sexual stage to the response to prostaglandins has also been demonstrated in goldfish (Stacey and Sorensen 1991) and treatment of juveniles with 17α-methyltestosterone increases the olfactory sensitivity to prostaglandins in other cyprinid species as well (Belanger et al. 2010). Gynogenetic females of Carassius auratus langsdorfii implanted with 11-ketotestosterone showed male spawning behavior to PGF2α-injected females (Kobayashi and Nakanishi 1999). It has also been shown that the olfactory sensory crypt cells, which have been suggested to detect sex pheromones in crucians, increase in numbers during the spawning period (Hamdani and Døving 2006; Lastein et al. 2006; Hamdani et al. 2008). The lateral bundles of the medial olfactory tract leading signals from the olfactory bulbs to the brain are important for the crucian male reproductive behavior to prostaglandin-injected females (Weltzien et al. 2003; Kawaguchi et al. 2014).
Behavior and physiological responses in single mature crucian males to 17,20β-P were studied in a fluviarium, which is an artificial stream with laminar flow of aerated tap water (Bjerselius et al. 1995a). The behavior of one male at the time was recorded during the night (00.00–06.00) with a video computer system in IR light (> 750 nm). The test area was divided in two halves, one with low concentration of the hormone added (nominal 10-11 M) and the other half with only tap water and the ethanol carrier. Tests were also run with regressed males with no running milt. The supply of the hormone shifted sides from one-half of the test area to the other every 90 min. After the test, blood plasma levels of the gonadotropin LH (former called GtH-II) were very high in mature males exposed to 17,20β-P, but not in regressed males or males only exposed to ethanol. This shows that 17,20β-P had a priming effect also in mature crucian males which is well in accordance with previous studies with goldfish (Kobayashi et al. 1986a, b; Dulka et al. 1987; Sorensen et al. 1989). Interestingly, the mature males spent a significantly longer time in the half with only the ethanol carrier. This was not observed in regressed males. They showed neither preference nor avoidance of the stream with 17,20β-P added. We suggest that the primed males, after having been stimulated by preovulatory females, continued searching for ovulating females that release PGF2α and other prostaglandins and are ready to spawn. An identical experiment with goldfish also showed that mature males spent less time on the side with 17,20β-P and had increased blood plasma levels of LH compared to control tests with no hormone added (Bjerselius et al. 1995b). The mature goldfish males, like crucians males, were more active and swam a longer distance when 17,20β-P was added. The results suggest that crucian carp and goldfish probably use the same hormonal pheromone system.
In the following experiments, hormonal effects in crucian males were studied under field conditions (Olsén et al. 2006). Crucian carp were, during the spawning season, June, trapped in a pond and kept separately by sex in floating net pens (Figs. 9, 10). Males were selected by the presence of breeding tubercles (Wiley and Collette 1970) and the presence of milt. Females had swollen abdomen that indicates complete vitellogenesis. Females injected with a solution of a gonadotropin-releasing analog and a dopamine antagonist (OvaprimTM Syndel induces LH surge and ovulation) and added to male pens induced LH blood plasma levels at all sample times (5, 9, 13 and 17 h with females) and increased milt volumes up to 10 times (9, 13 and 17 h) in males (Fig. 11). Females that were not injected with Ovaprim neither changed male LH blood plasma levels nor increased the strippable milt volumes. Females that ovulated spontaneously also induced increased LH levels and milt volumes. Further, the presence of females injected with the goldfish hormonal pheromone 17,20β-P resulted in increased LH levels and milt volumes 9 hours later in males (Fig. 12). The results suggest that crucian carp and goldfish use the same preovulatory steroid(s). Later, we demonstrated that preovulatory female rudd (Scardinius erythrophthalmus), a native cyprinid species within subfamily Leuciscinae, induced endocrine and gonadal responses in conspecific males and increased milt volumes and plasma levels of 17,20β-P (Stacey et al. 2012), but rudd females did not induce any responses in crucian males, which suggests that the female priming odors are species specific. There can be blends of species-specific body odors added to hormonal pheromones (Levesque et al. 2011; Lim and Sorensen 2011).
Experimental cages in a pond just outside Uppsala. The pond contains only crucian carp. The cages (2 × 1 × 0.6 m; LWD) were floating and open topped made of PVC tubing cover and 6 mm knotless nylon netting. Initially, males and females were held separately in cages covered by a floating mat of Potamogeton spp. to reduce stress. To establish experimental cages, males were selected from the holding cages and placed in cages of the same size and left undisturbed for 1–3 days. Experimental cages were not covered with Potamogeton. Swollen females (indicating complete vitellogenesis) injected with Ovaprim, composed of GnRH analog and dopamine antagonist (Syndel, Vancouver, BC, Canada), were placed with the males. Males were kept with Ovaprim-injected or untreated females for 0 h, 5 h, 9 h, 13 h or 17 h. After the exposure time, males were anesthetized and blood sampled from the caudal vasculature by syringe. This was followed by sampling of milt with hematocrit tubes. Zero hour males were simply removed from the experimental cages. To check if ovulation and spawning occurred before sampling of males, several artificial spawning substrates were tied in the corners of all cages
Effects of exposures to Ovaprim-injected and untreated females on LH (mean + SEM) b, and milt volumes (mean + SEM) in male crucian carp, a. Males kept in all-male groups (clear bars at 0 h), kept with untreated nonovulatory females (clear bars at 5–17 h), kept with ovaprim-injected females (black bars) or with spontaneously ovulating females (gray bars). Significant differences from control (nonovulatory female) group is indicated by *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Figure adapted from Olsén et al. (2006). Reproduced with the permission of General and Comparative Physiology. For further information, see Fig. 9
Effects of 10 h exposure to 17,20βP-injected females on LH (mean + SEM) and milt volumes (mean + SEM) in male crucian carp. Control males were placed with oil-injected females. Significant difference between the treatments: *P < 0.05, ****P < 0.0001. Figure from Olsén et al. (2006). Reproduced with the permission of General and Comparative Physiology
Chemical senses and foraging. Fish depend on chemoreception, vision and mechanoreception to different degrees during foraging (e.g., Ringler 1979). Odors often have arousal effect and induce food search behavior, followed by the location of food items by vision and, finally, taste and texture determine whether the fish will accept the food (e.g., Jones 1992; Kasumyan 2012; Løkkeborg et al. 2014). Crucians have received interest concerning the importance of the olfactory sense in feeding behavior, i.e., biting, snapping, mouth opening and vertical posture, when stimulated with food odor (Hamdani et al. 2001a, b). These studies evaluated the significance of certain types of olfactory sensory cells (with microvilli) in feeding behavior and their projection to the lateral olfactory tract (from the olfactory bulb to the telencephalon). Both the olfactory and gustatory senses in fish are sensitive to free amino acids and there are differences between species. Extracts of mussels are strong feeding stimulants in some bottom feeding carnivorous species and increase their growth rate when added to artificial feed (Mackie 1982). It has been suggested that Baltic Sea blue mussels (Mytilus edulis) should be an important feed component in aquaculture (e.g., Vidakovic et al. 2015). Mussels contain, in addition to amino acids important in protein synthesis, physiological active compounds such as betaine (trimethylglycine, osmolyte) that stimulates feeding in some fish species (Kasumyan and Døving 2003). Crucians are generalist feeders (omnivorous) and show plastic diet selection and their diet includes zooplankton, benthic insect larvae and plant and detritus materials (e.g., Prejs 1973; Lelek 1987; Penttinen and Holopainen 1992). A recent study also suggests that small molluscs are on the menu (Harper et al. 2021). The diversity of Mollusca decreased in fish empty ponds after the introduction of crucians. As in the closely related goldfish, crucians show a complex pattern of mouth handling of big food particles which is a post-capture selection process between food and non-food particles (Lamb and Finger 1995). In goldfish, the handling time is completed in 3–5 s with unflavored and 30–60 s with moderately aversive stimuli in pellets. The handling time is dependent on the size of crucians and their prey (Paszkowski et al. 1989). Dry mass ingested per unit of handling time strongly increased with fish size. The handling time was 90–100 s with the largest fish and prey length.
We have recently studied foraging and feeding stimulants in crucians. In our study with crucians, we used mouth handling time as an observation variable to be an indicator of palatability of agar pellets flavored with extracts and free amino acids (Olsén and Lundh 2016) (Fig. 13). Water extracts of their feed ®Hikari Sinking Wheat-Germ pellets, blue mussel meat meal and a cyprinid attractant (Swedbait, Uppsala, Sweden) were included in agar pellets and the testing procedure was adopted from the method described by Kasumyan and Morsi (1996) and Kasumyan (2012). Only extracts of Hikari stimulated mouth handling, but no stimulating effect was observed with extract of blue mussel meal or the commercial cyprinid attractant (Figs. 14, 15). The mussel extract had much higher concentrations of the free L-amino acids analyzed compared to Hikari and Swedbait extracts that had the lowest concentrations. There was no difference in amino acid rank order between the Hikari and mussel extracts. Based on the gustatory stimulatory effects in common carp and goldfish, five amino acids (l-alanine, l-arginine, glycine, l-leucine, l-arginine) were chosen to compose a mixture (Kasumyan and Døving 2003). A total concentration of 96 mM increased handling time compared to agar pellets with only water and the food dye Brown HT (E155). A 20 times lower concentration led to no increase in handling time. A mixture of l-alanine, l-arginine and glycine (tot 80 mM) and l-alanine (128 mM) on its own increased handling time (Fig. 16). The stimulating effect of l-alanine was significantly lower than pellets with Hikari extract (Fig. 17). Blue mussel extract did not stimulate feeding, though the high concentrations of amino acids indicate that the extract contained molecules that act as feeding deterrents in crucians. Compounds acting as feed stimulants and deterrents vary between species due to natural food choice (Kasumyan and Døving 2003). In another study, we found that water solutions of extracts of Hikari induced snapping behavior (olfaction), but mussel extracts had no effect (Olsén et al. 2018). Solutions of five amino acid with high concentrations in Hikari and mussel extracts produced no significant increase in snapping. In a previous study, amino acids did not induce any change in swimming activity or snapping behavior in crucians, only foraging movements with their heads directed to the bottom (Kruzhalov 1990). The test buds present on the ventral side of the head and lateral side of the body in crucians might be important for this bottom head behavior (Gomahr et al. 1992). The results suggest that free amino acids have minor influence in stimulating foraging in crucians and there is another potent molecule or molecules involved.
A food pellet punched out from a Petri dish with an aluminum tube with inner diameter of 5 mm. The agar pellet was collected with a stainless needle and carefully put in a test aquarium. The size of the agar pellet was about the same size as crucians’ ordinary feed, Hikari pellets. Food dye Brown HT (E155) was used to give the agar pellet a brownish color resembling the Hikari pellets
Comparisons in mouth handling time with agar pellets containing only water extract (control), extracts of Swedbait or Hikari pellet meal. Repeated measures ANOVA revealed significant differences between the agar pellets (P < 0.05) and Dunnett’s multiple comparisons test revealed a significantly longer handling time with Hikari compared to control agar pellets and Swedbait agar pellets (P < 0.05). Figure from Olsén and Lundh (2016). Reproduced with permission of Aquaculture Reports
Crucian carp mouth handling time with two concentrations of mussel meal extracts, 5.5 g/300 ml water in a, and 25 g/300 ml water in b. There were no statistically significant differences. Figure from Olsén and Lundh (2016). Reproduced by the permission of Aquaculture Reports
Crucian carp mouth handling time with agar pellets containing two amino acid mixtures, one mixture with three amino acids (Ala, Arg, Gly, total 80 mM) and with two amino acids (Ser, Leu, total 16 mM). A repeated measure ANOVA revealed that there were differences in handling time between the three pellets (P < 0.05). The three amino acids led to significantly longer handling time compared with the control pellets, P < 0.05 (Dunnett’s multi-comparisons test). Figure from Olsén and Lundh (2016). Reproduced with the permission of Aquaculture Reports
Comparisons between the stimulants that resulted in an increased handling time compared to controls. The difference in handling time between the agar pellets with stimulants and the control agar pellets for each individual was used as an observation. One-way ANOVA revealed that there were differences among the stimulants, P < 0.05. The Tukey’s multi-comparison test showed that alanine led to significantly shorter handling time than Hikari extracts, P < 0.05. Hikari agar 1, N = 6; Hikari agar 2, N = 6; 5AA, N = 6; 3AA, N = 5; alanine, N = 9. Figure from Olsén and Lundh (2016). Reproduced with the permission of Aquaculture Reports
Conclusions. Crucian carp, Carassius carassius (L.), is a rather unknown fish that has received interest in science. Due to its hardiness, crucian carp has been used as pond fish and food source from the fifteenth century up to the late eighteenth century. There has always been a supply of fresh fish. Crucians are one of the few vertebrates that survives anoxia. Several studies have elucidated the physiological mechanisms behind this fascinating ability. Liver glycogen as winter energy source and ethanol as excretion product instead of lactate are two key components. The hypoxia and anoxia tolerance have resulted in lakes and ponds with only crucians present in great numbers. Compared to high-bodied crucians in lakes with piscivores, the pond populations are shallow-bodied and small. The increase in body height is induced by exposure to fish predators such as pike and perch eating crucians. The mechanisms behind the inducible defense is not fully known. It is known that the olfactory sense is involved. Crucians are sensitive to predations and, by increasing the height of the body, predatory fish will have problems in swallowing the prey. Crucian carp is also sensitive to competition and the closely related invasive gibel carp, Carassius gibelio, has become a threat to crucians. Gibel carp is not native to Europe, but has been introduced from Asia and used in aquaculture. Crucian carp has disappeared in locations where gibels are present. The two species hybridize and it is difficult to discriminate between hybrids and pure crucians. Genetic analyses are needed to determine the species identity. Crucians have also become a model species in studies of chemoreception, olfaction and taste. In addition to that, crucians are hardy and easy to keep in the laboratory, and the species is closely related to goldfish, Carassius auratus, the most studied fish in chemoreception and behavior. Studies in the laboratory and in the natural environment have shown that wild crucians and goldfish probably use the same sex hormonal pheromone system.
References
Adams CE (1994) The fish community of Loch Lomond, Scotland: its history and rapidly changing status. Hydrobiologia 290:91–103
Aho J, Holopainen IJ (2000) Batch spawning in crucian carp (Carassius carassius (L)) in mono- and multiple-species communities. Ann Zool Fennici 37:101–111
Andersson J, Johansson F, Söderlund T (2006) Interactions between predator- and diet-induced phenotypic changes in body shape of crucian carp. Proc R Soc B 273:431–437
Backström T, Winberg S (2017) Serotonin coordinates responses to social stress—what we can learn from fish. Front Neurosci 11:595
Bagge AM, Poulin R, Valtonen ET (2004) Fish population size, and not density, as the determining factor of parasite infection: case study. Parasitology 128:305–313
Belanger RM, Pachkowski MD, Stacey NE (2010) Methyltestosterone-induced changes in electro-olfactogram responses and courtship behaviors of cyprinids. Chem Senses 35:65–74
Belchenko LA, Kel OV (1991) Adaptation to hypoxia in crucian carp, Carassius carassius, and wild goldfish, Carassius auratus gibelio. J Ichthyol 32:114–124
Bernos TA, Jeffries KM, Mandrak NE (2020) Linking genomics and fish conservation decision making: a review. Rev Fish Biol Fish 30:587–604
Bernström J (1969) Ruda (Carassius carassius). Kulturhistoriskt lexikon för nordisk medeltid. KLFNM Band XIV Regnebrœt Samgäld:440–442 (in Swedish)
Bjerselius R, Olsén KH (1993) A study of the olfactory sensitivity of crucian carp (Carassius carassius) and goldfish (Carassius auratus) to 17 20ß-dihydroxy-4-pregnen-3-one and prostaglandin F2. Chem Senses 18:427–436
Bjerselius R, Olsén KH, Zheng WB (1995a) Endocrine, gonadal and behavioral responses of male crucian carp to the hormonal pheromone 17 20ß-dihydroxy-4-pregnen-3-one. Chem Senses 20:221–230
Bjerselius R, Olsén KH, Zheng WB (1995b) Behavioral and endocrinological response of mature male goldfish to the sex hormone 17 20ß-dihydroxy-4-pregnen-3-one in the water. J Exp Biol 198:747–754
Blažka P (1958) The anaerobic metabolism in fish. Physiol Zool 31:117–128
Bonow M, Svanberg I (2011) »Säj får jag dig bjuda ur sumpen en sprittande ruda»: en bortglömde läckerhet från gångna tiders prästgårdskök. In: Bonow M, Rytkönen P (eds) Gastronomins (politiska) geografi in the Swedish society for anthropology and geography´s yearbook Ymer 2011. MotalaGrafiska, Motala, pp 147–169 (in Swedish)
Bonow M, Svanberg I (2012) Uppländska ruddammar: ett bidrag till akvakulturens kulturhistoria. In: Liby H (ed) Uppland 2012. Uppland: årsbok för medlemmarna i Upplands fornminnesförening och hembygdsförbund. Upplands fornminnesförening förlag, Uppsala, pp 123–152 (in Swedish)
Bonow M, Svanberg I (2016) Historical Pond-Breeding of Cyprinids in Sweden and Finland. In: Bonow M, Olsén H, Svanberg I (eds) Historical Aquaculture in Northern Europe. Södertörn University Research Report 2016 (1). Södertörn University, Stockholm, pp 91–123
Bonow M, Svanberg I (2018) Förvarings- och odlingsdammar: Fiskdammar i det senmedeltida och tidigmoderna Västergötland. In: Lokrantz A (ed) Speglingar av vatten i Skara. Västergötlands Fornminnesförenings Tidskrift. Västergötlands fornminnesförening, Skara, pp 51–59 (in Swedish)
Bonow M, Cios S, Svanberg I (2016) Fishponds in the Baltic states: historical cyprinid culture in Estonia, Latvia and Lithuania. In: Bonow M, Olsén H, Svanberg I (eds) Historical aquaculture in Northern Europe. Södertörn University Research Report 2016 (1). Södertörn University, Stockholm, pp 139–156
Brönmark C, Miner JG (1992) Predator-induced phenotypic change in body morphology in crucian carp. Science 258:1348–1350
Brönmark C, Pettersson LB (1994) Chemical cues from piscivores induce a change in morphology in crucian carp. Oikos 70:396–402
Brönmark C, Paszkowski CA, Tonn WM, Hargeby A (1995) Predation as a determinant of size structure in populations of crucian carp (Carassius carassius) and tench (Tinca tinca). Ecol Freshw Fish 4:85–92
Busst GMA, Britton JR (2015) Quantifying the growth consequences for crucian carp Carassius carassius of competition from non-native fishes. Ecol Freshw Fish 24:489–492
Chivers DP, Zhao X, Brown GE, Marchant TA, Ferrari MCO (2008) Predator-induced changes in morphology of a prey fish: the effects of food level and temporal frequency of predation risk. Evol Ecol 22:561–574
Copp GH, Tarkan AS, Godard MJ, Edmonds NJ, Wesley KJ (2010) Preliminary assessment of feral goldfish impacts on ponds, with particular reference to native crucian carp. Aquat Invasions 5:413–422
Demeny F, Sipos S, Ittzes I, Lévai P, Bodó B, Urbányi B, Müller T (2009) Observations of the crucian carp (Carassius carassius) pond culture. In: Marković Z (ed) Proceedings of the IV International Conference “Fishery”, May 27–29, 2009. Reinforcement of Sustainable Aquaculture. University of Belgrade, Belgrade, pp 138–144
Domenici P, Turesson H, Brodersen J, Brönmark C (2008) Predator-induced morphology enhances escape locomotion in crucian carp. Proc R Soc B 275:195–201
Dulka JG, Stacey NE, Sorensen PW, Van Der Kraak GJ (1987) A steroid sex pheromone synchronizes male-female spawning readiness in the goldfish. Nature 325:251–253
Ekre R (2007) Kloster i Lödöse. In: Hagberg J (ed) Kloster och klosterliv in det medeltida Skara stift. Skara stiftshistoriska sällskap, Skara, pp 109–158 (in Swedish)
Ekström CU (1831) Fiskarne i Mörkö Skärgård. Kongl Vetenskaps-Academiens Handlingar för år 1830:142–204 (in Swedish)
Ekström CU (1839) Iakttagelser öfver formförändringen hos rudan (Cypr. Carassius Lin.). Kongl Vetenskaps-Academiens Handlingar för år 1838:212–225 (in Swedish)
Fabricius K (1904) En nordisk lensmands liv i det 15de århundrade. Hist Tidskrift 1904:273–300 (in Danish)
Fraser D, Adams CE (1997) A crucian carp Carassius carassius (L.) in Loch Rannoch, Scotland: further evidence of the threat posed to unique fish communities by introduction of alien fish species. Aquat Conserv 7:323–326
Gomahr A, Palzenberger M, Kotrschal K (1992) Density and distribution of external taste buds in cyprinids. Environ Biol Fishes 33:125–134
Greenberg LA, Paszkowski CA, Tonn WM (1995) Effects of prey species composition and habitat structure of foraging by two functionally distinct piscivores. Oikos 74:522–532
Gu Q, Wang S, Yuan H, Zhong H, Yang J, Yang C, Huang X, Xu X, Wang Y, Wei Z, Wang J, Liu S (2021) The phylogeographic relationships and the evolution history of Carassius auratus complex with a newborn homodiploid crucian carp-like fish (2nNCRC). BMC Genomics. https://doi.org/10.21203/rs.3.rs-604445/v1
Hamdani EH, Døving KB (2002) The alarm reaction in crucian carp is mediated by olfactory neurons with long dendrites. Chem Senses 27:395–398
Hamdani EH, Døving KB (2003) Sensitivity and selectivity of neurons in the medial region of the olfactory bulb to skin extract from conspecifics in crucian carp, Carassius carassius. Chem Senses 28:181–189
Hamdani EH, Døving KB (2006) Specific projection of the sensory crypt cells in the olfactory system in crucian carp, Carassius carassius. Chem Senses 31:63–67
Hamdani EH, Stabell OB, Alexander G, Døving KB (2000) Alarm reaction in the crucian carp is mediated by the medial bundle of the medial olfactory tract. Chem Senses 25:103–109
Hamdani EH, Kasumyan A, Døving KB (2001a) Is feeding behaviour in crucian carp mediated by the lateral olfactory tract? Chem Senses 26:1133–1138
Hamdani EH, Alexander G, Døving KB (2001b) Projection of sensory neurons with microvilli to the lateral olfactory tract indicates their participation in feeding behaviour in crucian carp. Chem Senses 26:1139–1144
Hamdani EH, Lastein S, Gregersen F, Døving KB (2008) Seasonal variations in olfactory sensory neurons—Fish sensitivity to sex pheromones explained? Chem Senses 33:119–123
Harper LR, Handley LL, Sayer CD, Read DS, Benucci M, Blackman RC, Hill MJ, Hänfling B (2021) Assessing the impact of the threatened crucian carp (Carassius carassius) on pond invertebrate diversity: A comparison of conventional and molecular tools. Mol Ecol 30:3252–3269
Hänfling B, Bolton P, Harley M, Carvalho GR (2005) A molecular approach to detect hybridisation between crucian carp (Carassius carassius) and non-indigenous carp species (Carassius spp. and Cyprinus carpio). Freshw Biol 50:403–417
Holopainen IJ, Hyvärinen H (1985) Ecology and physiology of crucian carp (Carassius carassius (L.)) in small Finnish ponds with anoxic conditions in winter. Verh Internat Verein Limnol 22:2566–2570
Holopainen IJ, Pitkänen AK (1985) Population size and structure of crucian carp (Carassius carassius (L.)) in two small, natural ponds in Eastern Finland. Ann Zool Fennici 22:397–406
Holopainen IJ, Hyvärinen H, Piironen J (1986) Anaerobic wintering of crucian carp (Carassius carassius L.) - II. Metabolic products. Comp Biochem Physiol A Physiol 83:239–242
Holopainen IJ, Tonn WM, Paszkowski CA (1997a) Tales of two fish: the dichotomous biology of crucian carp (Carassius carassius (L)) in northern Europe. Ann Zool Fennici 34:1–22
Holopainen IJ, Aho J, Vornanen M, Huuskonen H (1997b) Phenotypic plasticity and predator effects on morphology and physiology of crucian carp in nature and in the laboratory. J Fish Biol 50:781–798
Horoszewicz L (1973) Lethal and “disturbing” temperatures in some fish species from lakes with normal and artificially elevated temperature. J Fish Biol 5:165–181
Höglund E, Balm PH, Winberg S (2000) Skin darkening, a potential social signal in subordinate arctic charr (Salvelinus alpinus): the regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. J Exp Biol 203:1711–1721
Hufthammer AK, Moe D (2016) Chapter 5. Fishponds and aquaculture in historical times in Norway. In: Bonow M, Olsén H, Svanberg I (eds) Historical aquaculture in Northern Europe. Södertörn University Research Report 2016 (1). Södertörn University, Stockholm, pp 121–138
Hyvärinen H, Holopainen IJ (1983) Anaerobic metabolism and wintering of crucian carp (Carassius carassius L.). Proc Int Union Physiol Sci 15:257
Hyvärinen H, Holopainen IJ, Pironen J (1985) Anaerobic wintering of crucian carp (Carassius carassius L.) – I. Annual dynamics of glycogen reserves in nature. Comp Biochem Physiol A Physiol 82:797–803
Janson S, Wouters J, Bonow M, Svanberg I, Olsén KH (2015) Population genetic structure of crucian carp (Carassius carassius) in man-made ponds and wild populations in Sweden. Aquac Int 23:359–368
Jeffries DL, Copp GH, Handley LL, Olsén KH, Sayer CD, Hänfling B (2016) Comparing RADseq and microsatellites to infer complex phylogeographic patterns, an empirical perspective in the crucian carp Carassius carassius, L. Mol Ecol 25:2997–3018
Jeffries DL, Copp GH, Maes GE, Handley LL, Sayer CD, Hänfling B (2017) Genetic evidence challenges the native status of a threatened freshwater fish (Carassius carassius) in England. Ecol Evol 7:2871–2882
Johansson D, Nilsson GE, Døving KB (1997) Anoxia depression of light-evoked potentials in retina and optic tectum of crucian carp. Neurosci Lett 237:73–76
Johnston IA (1975) Anaerobic metabolism in the carp (Carassius carassius L.). Comp Biochem Physiol B 51:235–241
Johnston IA, Bernard LM (1983) Utilization of the ethanol pathway in carp following exposure to anoxia. J Exp Biol 104:73–78
Johnston IA, Maitland B (1980) Temperature acclimation in crucian carp, Carassius carassius L., morphometric analyses of muscle fibre ultrastructure. J Fish Biol 17:113–125
Jones KA (1992) Food search behaviour in fish and the use of chemical lures in commercial and sport fishing. In: Hara TJ (ed) Fish chemoreception. Fish and fisheries series 6. Chapman & Hall, London, pp 288–320
Jonsson L (1984) Djur i staden. Upplands fornminnesförenings tidskrift 50:88–94 (in Swedish)
Kagawa N, Mugiya Y (2000) Exposure of goldfish (Carassius auratus) to bluegills (Lepomis macrochirus) enhances expression of stress protein 70 mRNA in the brains and increases plasma cortisol levels. Zoolog Sci 17:1061–1066
Kamshilov IM, Kamshilov TB (2019) Functional properties of hemoglobin in bream Abramis brama and in crucian carp Carassius carassius at hypoxia. J Ichthyol 59:369–372
Karling S (1928) Vadstena klosterträdgård. Rig - Kulturhistorisk tidskrift 11:155–170 (in Swedish)
Karvonen A, Bagge AM, Valtonen ET (2005) Parasite assemblages of crucian carp (Carassius carassius) – is depauperate composition explained by lack of parasite exchange, extreme environmental conditions of host unsuitability? Parasitology 131:273–278
Kasprzak R, Ostaszewska T, Wagner B (2019) The effect of feeding commercial diets on the development of juvenile crucian carp (Carassius carassius, L.). Part 1: skeletal deformations. Aquac Nutr 25:78–87
Kasumyan AO (2012) The intraoral tactile reception and its interaction with the gustatory system in fish. Dokl Akad Nauk 447:579–581
Kasumyan AO, Døving KB (2003) Taste preferences in fishes. Fish Fish 4:289–347
Kasumyan AO, Morsi AM (1996) Taste sensitivity of common carp Cyprinus carpio to free amino acids and classical taste substances. J Ichthyol 36:391–403
Kawaguchi Y, Nagaoka A, Kitami T, Hayakawa Y, Kobayashi M (2014) Gender-typical olfactory regulation of sexual behavior in goldfish. Front Neurosci 8:91
Kellner M, Porseryd T, Porsch-Hällström I, Hansen SH, Olsén KH (2016) Waterborn citalopram has anxiolytic effects and increases locomotor activity in the three-spine stickleback (Gasterosteus aculeatus). Aquat Toxicol 173:19–28
Klemming GE (1866) Svenska medeltidens rimkrönikor 2. Nya eller Karlskrönikan. Början av unionsstriderna samt Karl Knutssons regering 1389–1452. Samlingar utgivna av Svenska fornskriftssällskapet. Serie 1, Svenska skrifter, 17, 2. P. A. Norstedt & Söner, Stockholm (in Swedish)
Kobayashi M, Nakanishi T (1999) 11-ketotestosoterone induces male-type sexual behavior and gonadotropin secretion in gynogenetic crucian carp, Carassius auratus langsdorfii. Gen Comp Endocrinol 115:178–187
Kobayashi M, Aida K, Hanyu I (1986a) Gonadotropin surge during spawning in male goldfish. Gen Comp Endocrinol 62:70–79
Kobayashi M, Aida K, Hanyu I (1986b) Pheromone from ovulatory female goldfish induces gonadotropin surge in males. Gen Comp Endocrinol 63:451–455
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Kottelat, Cornol & Freyhof, Berlin
Kovalchuk OM (2014) Bony fishes from the late Miocene and Pliocene strata of Popovo locality (Ukraine): Taxonomic changes and their paleoecological explanation. Vestn Zool 48:129–136
Kruzhalov NB (1990) Attraction and repellent reactions to amino acids by crucian carp, Carassius carassius. J Ichthyol 30:165–170
Kuljanishvili T, Mumladze L, Japoshvili B, Mustafayev N, Ibrahimov S, Patoka J, Pipoyan S, Kalous L (2021) The first unified inventory of non-native fishes of the south Caucasian countries, Armenia, Azerbaijan, and Georgia. Knowl Manag Aquat Ecosyst 422:32
Kullander SO, Nyman L, Jilg K, Delling B (2012) Nationalnyckeln till Sveriges flora och fauna. Strålfeniga fiskar. Actinopterygii. SLU ArtDatabanken, Uppsala (in Swedish and partly in English)
Lamb CF, Finger TE (1995) Gustatory control of feeding behavior in goldfish. Physiol Behav 57:483–488
Lappalainen M, Vinni M, Malinen T (2013) Consumption of crucian carp (Carassius carassius L., 1758) by stocked pike (Esox lucius L., 1758) in a lake with frequent winter anoxia. J Appl Ichthyol 29:1286–1291
Lastein S, Hamdani EH, Døving KB (2006) Gender distinction in neural discrimination of sex pheromones in the olfactory bulb of crucian carp, Carassius carassius. Chem Senses 31:69–77
Lastein S, Hamdani EH, Døving KB (2008a) Single unit responses to skin odorants from conspecifics and heterospecifics in the olfactory bulb in crucian carp Carassius carassius. J Exp Biol 211:3529–3535
Lastein S, Höglund E, Mayer I, Øverli Ø, Døving KB (2008b) Effects of antalarmin, a CRF receptor 1 antagonist, on fright reaction and endocrine stress response in crucian carp (Carassius carassius). J Comp Physiol A 194:1007–1012
Laurila S, Piironen J, Holopainen IJ (1987) Notes on egg development, larval and juvenile growth of crucian carp (Carassius carassius (L.)). Ann Zool Fennici 24:315–321
Lelek A (1987) Threatened fishes of Europe. In: The European Committee for the Conservation of Nature and Natural Resources, Council of Europe (ed) The freshwater fishes of Europe, vol 9. Aula-Verlag, Wiesbaden, Germany, pp 168–170
Lenkiewicz S (1964) Temperature preferendum of some freshwater fishes. Folia Biol 12:95–140
Lepiksaar J (1969) Djurrester från det medeltida Ny Varberg. Fynd från karmeliterklostret ca 1470–1530. Varbergs Museums Årsbok 16:73–102 (in Swedish)
Levesque HM, Scaffidi D, Polkinghorne CN, Sorensen PW (2011) A multi-component species identifying pheromone in the goldfish. J Chem Ecol 37:219–227
Libosvársky J (1962) Zur palaeoboreal Verbreitung der Gattung Carassius. Zool Jb Syst 90:197–210
Lim H, Sorensen PW (2011) Polar metabolites synergize the activity of prostaglandin F2αin a species-specific hormonal sex pheromone released by ovulated common carp. J Chem Ecol 37:695–704
Løkkeborg S, Siikavuopio SI, Humborstad OB, Unte-Palm AC, Ferter K (2014) Towards more efficient longline fisheries: fish feeding behaviour, bait characteristics and development of alternative baits. Rev Fish Biol Fish 24:985–1003
Lõugas L (1999) Postglacial development of fish and seal faunas in the Eastern Baltic water system. In: Benecke N (ed) The holocene history of the European vertebrate fauna. Modern aspects of research. Workshop 6th to 9th April 1998, Berlin. Archäologie in Eurasien 6. Verlag Marie Leidorf GmbH, Rahden, pp 185–200
Lusková V, Lusk S, Halačka K, Vetesník L (2010) Carassius auratus gibelio—the most successful invasive fish in waters of the Czech Republic. Russ J Biol Invasions 1:176–180
Łączyńska B, Palińska-Żarska K, Nowosad J, Biłas M, Krejszeff S, Müller T, Kucharczuk D, Żarski D (2016) Effect of age, size and digestive tract development on weaning effectiveness in crucian carp, Carassius carassius (Linneaus, 1758). J Appl Ichthyol 32:866–872
Mackie AM (1982) Identification of the gustatory feeding stimulants. In: Hara TJ (ed) Fish chemoreception. Fish and Fisheries Series 6. Chapman & Hall, London, pp 275–291
Makowiecki D (1999) Some aspects of studies on the evolution of fish faunas and fishing in prehistoric and historic times in Poland. In: Benecke N (ed) The holocene history of the European vertebrate fauna. Modern aspects of research. Workshop 6th to 9th April 1998, Berlin. Archäologie in Eurasien 6. Verlag Marie Leidorf GmbH, Rahden, pp 171–184
Makowiecki D (2000) Catalogue of subfossil fish remains from Poland. Archaeofauna 9:133–149
Melfors E (1991) Ivar Axelsson Totts räkenskapsbok för Gotland 1485–1487. Acta Eruditorum Gotlandia. Actum VII. Gotlands Fornsal, Visby (in Swedish)
Mezhzherin SV, Kulish AV, Kokodiy SV (2019) Structure and dynamics of crucians’ settlements (Cyprinoformes, Carassius) in water systems of Eastern Ukraine. Vestn Zool 53:269–284
Mickiewics M, Wołos A (2012) Economic ranking of the importance of fish species to lake fisheries stocking management in Poland. Arch Pol Fish 20:11–18
Murphy CA, Stacey NE, Corkum LD (2001) Putative steroidal pheromones in the round goby, Neogobius melanstomus: Olfactory and behavioral responses. J Chem Ecol 27:443–470
Myszkowski L, Kamiński R, Quiros M, Stanny LA, Wolnicki J (2002) Dry diet-influenced growth, size variability, condition and body deformities in juvenile crucian carp Carassius carassius L. reared under controlled conditions. Arch Pol Fish 10:51–61
Nielsen SV, Kellner M, Henriksen PG, Olsén H, Hansen SH, Baatrup E (2018) The psychoactive drug Escitalopram affects swimming behaviour and increases boldness in zebrafish (Danio rerio). Ecotoxicology 27:485–497
Nilsson A (1939) Fiskodling i Skåne i äldre tid. Skånes hembygdsförbunds årsbok 1939:86–99 (in Swedish)
Nilsson GE, Rosén P, Johansson D (1993) Anoxic depression of spontaneous locomotor activity in crucian carp quantified by a computerized imaging technique. J Exp Biol 180:153–162
Nilsson P (2010) Bortom åker och äng: förekomst och betydelse av kvarnar, fiske, humle- och fruktodlingar enligt de äldre geometriska kartorna (ca 1630–1650). Acta Universitatis Agriculturae Sueciae. Sveriges lantbruksuniv ersitet, Uppsala
Nilsson PA, Brönmark C, Pettersson LB (1995) Benefits of a predator-induced morphology in crucian carp. Oecologia 104:291–296
Nilsson S (1855) Ruda (Cyprinus carassius Lin.). In: Nilsson S (ed) Skandinavisk Fauna. Fjerde delen: Fiskarna. CWK Gleerups Förlag, Lund, pp 290–296 (in Swedish)
Nordeide SW, Hufthammer AK (2009) Fishponds as garden features: the example from the Archbishop’s Palace, Trondheim. In: Moreland JP, Mercuri AM (eds) Plants and culture: seeds of the cultural heritage of Europe. Edipuglia, Ravello, pp 277–282
Nordström T, Dahlander M (1908) Örebro slotts byggnadshistoria. Lindhska bokhandeln, Örebro (in Swedish)
O’Connor KI, Metcalfe NB, Taylor AC (1999) Does darkening signal submission in territorial contests between juvenile Atlantic salmon, Salmo salar? Anim Behav 58:1269–1276
Olsén KH, Lundh T (2016) Feeding stimulants in an omnivorous species, crucian carp Carassius carassius (Linnaeus 1758). Aquac Rep 4:66–73
Olsén KH, Sawisky GR, Stacey NE (2006) Endocrine and milt responses of male crucian carp (Carassius carassius L.) to periovulatory females under field conditions. Gen Comp Endocrinol 149:294–302
Olsén KH, Ask K, Olsén H, Porsch-Hällström I, Hallgren S (2014) Effects of the SSRI citalopram on behaviours connected to stress and reproduction in Endler guppy, Poecilia wingei. Aquat Toxicol 148C:113–121
Olsén KH, Sukovich N, Backman J, Lundh T (2018) Chemical foraging stimulation in the omnivorous species crucian carp Carassius carassius (Linnaeus 1758). Aquac Rep 12:36–42
Öhman J, Buffam I, Englund G, Blom A, Lindgren E, Laudon H (2006) Associations between water chemistry and fish community composition: a comparison between isolated and connected lakes in northern Sweden. Freshw Biol 51:510–522
Øksnevad SA, Poleo ABS, Øsbye K, Heibo K, Andersen RA, Vøllestad RA (1995) En ny teori om karussens innvandring og utbredelse i Norge. Fauna 48:123–127 (in Norwegian)
Papoušek L, Vetešnik K, Halačka V, Lusková M, Humpl M, Mendel J (2008) Identification of natural hybrids of gibel carp Carassius auratus gibelio (Bloch) and crucian carp Carassius carassius (L.) from lower Dyje River floodplain (Czech Republic). J Fish Biol 72:1230–1235
Paszkowski CA, Tonn WM, Holopainen IJ (1989) An experimental study of body size and food size relations in crucian carp, Carassius carassius. Environ Biol Fishes 24:275–286
Paszkowski CA, Tonn WM, Piironen J, Holopainen IJ (1990) Behavioral and population-level aspects of intraspecific competition in crucian carp. Ann Zool Fennici 27:77–85
Penttinen OP, Holopainen IJ (1992) Seasonal feeding activity and ontogenetic dietary shifts in crucian carp, Carassius carassius. Environ Biol Fishes 24:275–286
Pettersson LB, Brönmark C (1993) Trading off safety against food: state dependent habitat choice and foraging in crucian carp. Oecologia 95:353–357
Pettersson LB, Nilsson PA, Brönmark C (2000) Predator recognition and defence strategies in crucian carp, Carassius carassius. Oikos 88:200–212
Pettersson LB, Andersson K, Nilsson K (2001) The diel activity of crucian carp, Carassius carassius, in relation to chemical cues from predators. Environ Biol Fishes 61:341–345
Piironen J, Holopainen IJ (1986) A note on seasonality in anoxia tolerance of crucian carp (Carassius carassius (L.)) in the laboratory. Ann Zool Fennici 23:335–338
Piironen J, Holopainen IJ (1988) Length structure and reproductive potential of crucian carp (Carassius carassius (L.)) populations in some small forest ponds. Ann Zool Fennici 25:203–208
Poléo ABS, Øxnevad SA, Østbye K, Heibo E, Andersen A, Vøllestad A (1995) Body morphology of crucian carp Carassius carassius in lakes with or without piscivorous fish. Ecography 18:225–229
Prejs A (1973) Experimentally increased fish stocks in the pond like lake Lake Warniak. IV. Feeding of introduced and autochthonous non-predatory fish. Ekol Pol 21:464–505
Rasmussen H (1959) Fiskedamme of fiskeopdrœt. Kulturhistoriskt lexikon för nordisk medeltid 4:308–309 (in Danish)
Ringler NH (1979) Prey selection by benthic feeders. In: Stoud R, Clepper H (eds) Predatory-prey systems in fishery management. Proceedings of the International Symposium on predatory-systems in fish communities and their role in fisheries management. Sport Fishing Institute, Washington, pp 219–229
Rylková K, Kalous L, Bohlen J, Lamatsch DK, Petrtýl M (2013) Phylogeny and biogeographic history of the cyprinid fish genus Carassius (Teleostei: Cyprinidae) with focus on natural and anthropogenic arrivals in Europe. Aquaculture 380–383:13–20
Sayer CD, Emson D, Patmore IR, Greaves HM, West WP, Payne J, Davies GD, Tarkan AS, Wiseman G, Cooper B, Grapes T, Cooper G, Copp GH (2020) Recovery of the crucian carp Carassius carassius (L.): approach and early results of an English conservation project. Aquat Conserv 30:2240–2253
Scott AP, Ellis T (2007) Measurement of fish steroids in water – a review. Gen Comp Endocrinol 153:392–400
Shinohara Y, Kobayashi M (2020) Sexual bipotentiality of the olfactory pathway for sexual behavior in goldfish. Fish Sci 86:819–827
Sikorska J, Kondera E, Kamiński R, Ługowska K, Witeska M, Wolnicki J (2018) Effect of four rearing water temperatures on some performance parameters of larval and juvenile crucian carp, Carassius carassius, under controlled conditions. Aquac Res 49:3874–3880
Sollid J, De Angelis P, Gundersen K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J Exp Biol 206:3667–3673
Sorensen PW, Hara TJ, Stacey NE, Goetz FW (1988) F-prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex pheromone in goldfish. Biol Reprod 39:1039–1050
Sorensen PW, Chamberlain KJ, Stacey NE (1989) Differing behavioural and endocrinological effects of two female sex pheromones on male goldfish. Horm Behav 23:317–332
Stabell OB, Lwin MS (1997) Predator-induced phenotypic changes in crucian carp are caused by chemical signals from conspecifics. Environ Biol Fishes 49:145–149
Stabell OB, Faeravaag AC, Tuvikene A (2010) Challenging fear: chemical alarm signals are not causing morphology changes in crucian carp (Carassius carassius). Environ Biol Fishes 89:151–160
Stacey NE (2015) Chapter 3. Hormonally-derived pheromones in teleost fish. In: Sorensen PW, Wisenden BD (eds) Fish pheromones and related cues. John Wiley and Sons, Inc., Hoboken, pp 33–88
Stacey NE, Sorensen PW (1991) Function and evolution of fish hormonal pheromones. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes: phylogenetic and biochemical perspectives. Vol. 1. Elsevier, New York, pp 109–135
Stacey NE, Sorensen PW (2006) Chapter 9. Reproductive pheromones. In: Sloman KA, Wilson RW, Balshine S (eds) Behaviour and physiology of fish. Fish Physiology. Vol 24. Academic Press, London, pp 359–412
Stacey NE, Sorensen PW, Van Der Kraak GJ, Dulka JG (1989) Direct evidence that 17α,20β-dihydroxy-4-pregnen-3-one functions as a goldfish primer pheromone: preovulatory release is closely associated male endocrine responses. Gen Comp Endocrinol 75:62–70
Stacey NE, Van Der Kraak GJ, Olsén KH (2012) Male primer endocrine responses to preovulatory female cyprinids under natural conditions in Sweden. J Fish Biol 80:147–165
Svanberg I, Cios S (2014) Petrus Magni and the history of fresh-water aquaculture in the later Middle Ages. Arch Nat Hist 41:124–130
Svanberg I, Bonow M, Olsén H (2012) Fish ponds in Scania, and Linnaeus’s attempt to promote aquaculture in Sweden. Svenska Linnésällskapets Årsskrift 2012:83–98
Szczerbowski JA, Szczerbowski AJ (2002) Carassius carassius (Linnaeus, 1758). In: Bănărescue PM, Paepke HJ (eds) The freshwater fishes of Europe. Vol 5/III. Aula-Verlag, Wiebelsheim, pp 43–78
Tagesson G (2000) Var låg Linköpings franciskanerkonvent? Fornvännen 95:217–236 (in Swedish)
Takada M, Tachihara K, Kon T, Yamamoto G, Iguchi K, Miya M, Nishida M (2010) Biogeography and evolution of the Carassius auratus-complex in East Asia. BMC Evol Biol 10:7
Targońska K, Żarski D, Müller T, Krejszeff S, Kozłowski K, Demény F, Urbányi B, Kucharczyk D (2012) Controlled reproduction of the crucian carp Carassius carassius (L.) combining temperature and hormonal treatments in spawners. J Appl Ichthyol 28:849–899
Tarkan AS, Almeida D, Godard MJ, Gaygusuz Ö, Rylands M, Sayer C, Zieba G, Copp G (2016) A review and meta-analysis of growth and life-history traits of a declining European freshwater fish, crucian carp Carassius carassius. Aquat Conserv 26:212–224
Tonn WM, Paszkowksi CA, Holopainen IJ (1989) Responses of crucian carp populations to differential predation pressure in a manipulated pond. Can J Zool 67:2841–2849
Tonn WM, Paszkowksi CA, Holopainen IJ (1992) Piscivory and recruitment: mechanisms structuring prey populations in small lakes. Ecology 73:951–958
Tonn WM, Holopainen IJ, Paszkowski CA (1994) Density-dependent effects and the regulation of crucian carp populations in single-species ponds. Ecology 75:824–834
Tóth B, Várkonyi E, Hidas A, Meleg EE, Váradi L (2005) Genetic analysis of offspring from intra- and interspecific crosses of Carassius auratus gibelio by chromosome and RAPD analysis. J Fish Biol 66:784–797
Vetemaa M, Eschbaum R, Albert A, Saat T (2005) Distribution, sex ratio and growth of Carassius gibelio (Bloch) in coastal and inland waters of Estonia (north-eastern Baltic Sea) J Appl Ichthyol 21:287–291
Vidakovic A, Langeland M, Sundh H, Sundell K, Olstorpe M, Vielma J, Kiessling A, Lundh T (2015) Evaluation of growth performance and intestinal function in Arctic charr (Salvelinus alpinus) fed yeast (Saccharomyces cerevisiae) fungi (Rhizopus oryzae) and blue mussel (Mytilus edulis). Aquac Nutr 22:1348–1360
Vinterstare J, Hulthén K, Nilsson PA, Sköld HN, Brönmark C (2020) Experimental manipulation of perceived risk and cortisol generates contrasting trait trajectories in plastic crucian carp. J Exp Biol 223:jeb213611
Vinterstare J, Brönmark C, Nilsson PA, Langerhans RB, Berglund O, Örjes J, Brodin T, Fick J, Hulthén K (2021) Antipredator phenotype in crucian carp altered by a psychoactive drug. Ecol Evol 11:9435–9446
Volta P, Jeppesen E, Sala P, Galafassi S, Foglini C, Puzzi C, Winfield IJ (2018) Fish assemblages in deep Italian subalpine lakes: history and present status with an emphasis on non-native species. Hydrobiologia 824:255–270
Vornanen M, Paajanen V (2004) Seasonality of dihydropyridine receptor binding in the heart of an anoxia-tolerant vertebrate, the crucian carp (Carassius carassius L.). Am J Physiol Regul Integr Comp Physiol 287:R1263–R1269
Vornanen M, Paajanen V (2006) Seasonal changes in glycogen content and Na+-K+-ATPase activity in the brain of crucian carp. Am J Physiol Regul Integr Comp Physiol 291:R1482–R1489
Vornanen M, Stecyk J, Nilsson GE (2009) The anoxia-tolerant crucian carp (Carassius carassius L.). In: Richards JG, Farrel AP, Brauner CJ (eds) Hypoxia. Vol 27. Elsevier, Amsterdam, pp 397–441
Vornanen M, Asikainen J, Haverinen J (2011) Body mass dependence of glycogen stores in the anoxia-tolerant crucian carp (Carassius carassius L.). Naturwissenschaften 98:225–232
Vøllestad LA, Varreng K, Poléo ABS (2004) Body depth variation in crucian carp Carassius carassius: an experimental individual-based study. Ecol Freshw Fish 13:197–202
Walker RM, Johansen PH (1977) Anaerobic metabolism in goldfish (Carassius auratus). Can J Zool 55:1304–1311
Weltzien FA, Höglund E, Hamdani EH, Døving KB (2003) Does the lateral bundle of the medial olfactory tract mediate reproductive behavior in male crucian carp? Chem Senses 28:293–300
Wheeler A (2000) Status of the crucian carp, Carassius carassius (L.) in the UK. Fish Manag Ecol 7:315–322
Wiley ML, Colette BB (1970) Breeding tubercles and contact organs in fishes: their occurrence, structure, and significance. Bull Am Mus Nat Hist 143:145–216
Willmer P, Stone G, Johnston I (2005) Environmental physiology of animals. Second Edition. Blackwell Publishing, Hoboken
Winfield IJ, Durie NC (2004) Fish introductions and their management in the English Lake District. Fish Manag Ecol 11:195–201
Winfield IJ, Fletcher JM, James JB (2011) Invasive fish species in the largest lakes of Scotland, Northern Ireland, Wales and England: the collective UK experience. Hydrobiologia 660:93–103
Wisenden BD, Pogatshnik J, Gibson D, Bonacci L, Schumacher A, Willett A (2008) Sound the alarm: learned association of predation risk with novel auditory stimuli by fathead minnows (Pimephales promelas) and glowlight tetras (Hemigrammus erythrozonus) after single simultaneous pairings with conspecific chemical alarm cues. Environ Biol Fishes 81:141–147
Wouters J, Janson S, Lusková V, Olsén KH (2012) Molecular identification of hybrids of crucian carp Carassius carassius and the invasive, gibel carp Carassius auratus gibelio in Swedish waters. J Fish Biol 80:2595–2604
Yamamoto G, Takada M, Kei'ichiro I, Mutsumi N (2010) Genetic constitution and phylogenetic relationships of Japanese crucian carps (Carassius). Ichthyol Res 57:215–222
Zabilska-Kunek M, Makowiecki D, Kabaciński J (2016) Mesolithic fishery in the Polish Lowland. Fish remains from site 7 at Krzyż Wielkopolski, Poland. Environ Archaeol 21:317–324
Żarski D, Targońska K, Krejszeff S, Kwiatkowski M, Kupren K, Kucharczyk D (2011) Influence of stocking density and type of feed on the rearing of crucian carp, Carassius carassius (L.), larvae under controlled conditions. Aquac Int 19:1105–1117
Żarski D, Horváth A, Bernáth G, Palińska-Żarska K, Krejszeff S, Müller T, Kucharczyk D (2014) Application of different activating solutions to in vitro fertilization of crucian carp, Carassius carassius (L.), eggs. Aquac Int 22:173–184
Acknowledgments
School of Natural Sciences, Södertörn University, has given financial support to finish the work of this manuscript. Publishers and authors have given permissions to reuse figures.
Funding
Open access funding provided by Södertörn University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Olsén, K.H., Bonow, M. Crucian carp (Carassius carassius (L.)), an anonymous fish with great skills. Ichthyol Res 70, 313–331 (2023). https://doi.org/10.1007/s10228-022-00892-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-022-00892-z