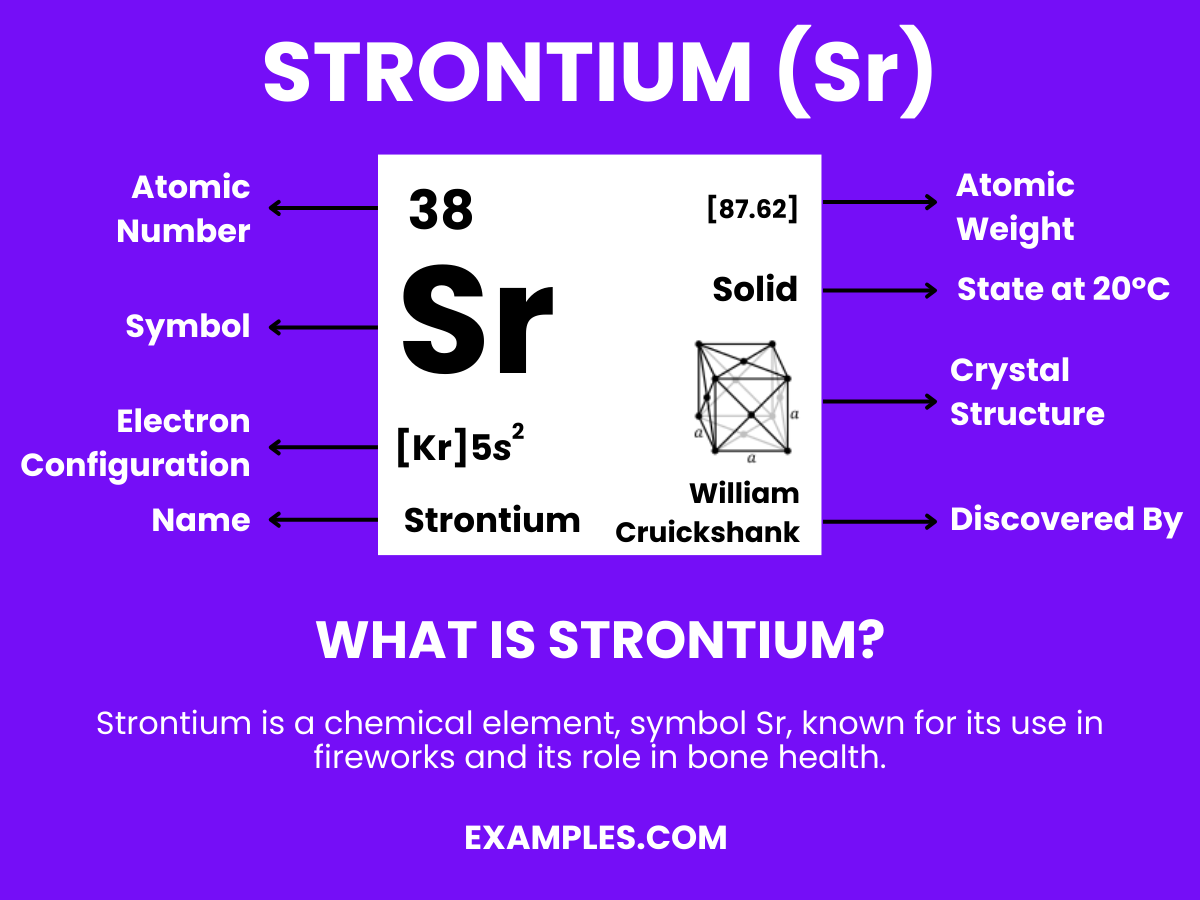
Strontium
Strontium, a key player in the periodic table, is more than just a chemical element. It’s a gateway to a world of scientific exploration and understanding. For teachers looking to enrich their classroom, Strontium offers a unique opportunity to delve into the realms of chemistry and physics. This guide will illuminate the intricate details of Strontium, from its fundamental properties to its varied applications in everyday life and scientific research. As educators, understanding Strontium’s role in the world around us not only enhances our knowledge but also empowers us to inspire our students with real-world examples.
What is Strontium?
Strontium is an element found in the Earth’s crust. Known for its bright red flames in fireworks and flares, it’s much more than a spectacle. In its pure form, Strontium is a silvery-white metal that reacts with air and water. It has applications ranging from making TV screens to strengthening bones in osteoporosis treatments. Understanding Strontium’s properties and uses helps us appreciate its role in both nature and technology. For teachers, this knowledge is invaluable in making chemistry relatable and engaging for students.
Other Alkaline Earth Metals
| Beryllium(Be) |
| Magnesium(Mg) |
| Calcium(Ca) |
| Barium(Ba) |
| Radium(Ra) |
Strontium Formula
- Formula: Sr
- Composition: A single strontium atom.
- Bond Type: Strontium forms ionic bonds, influenced by its two valence electrons.
- Molecular Structure: Soft, silvery metal that tarnishes yellowish or pinkish in air.
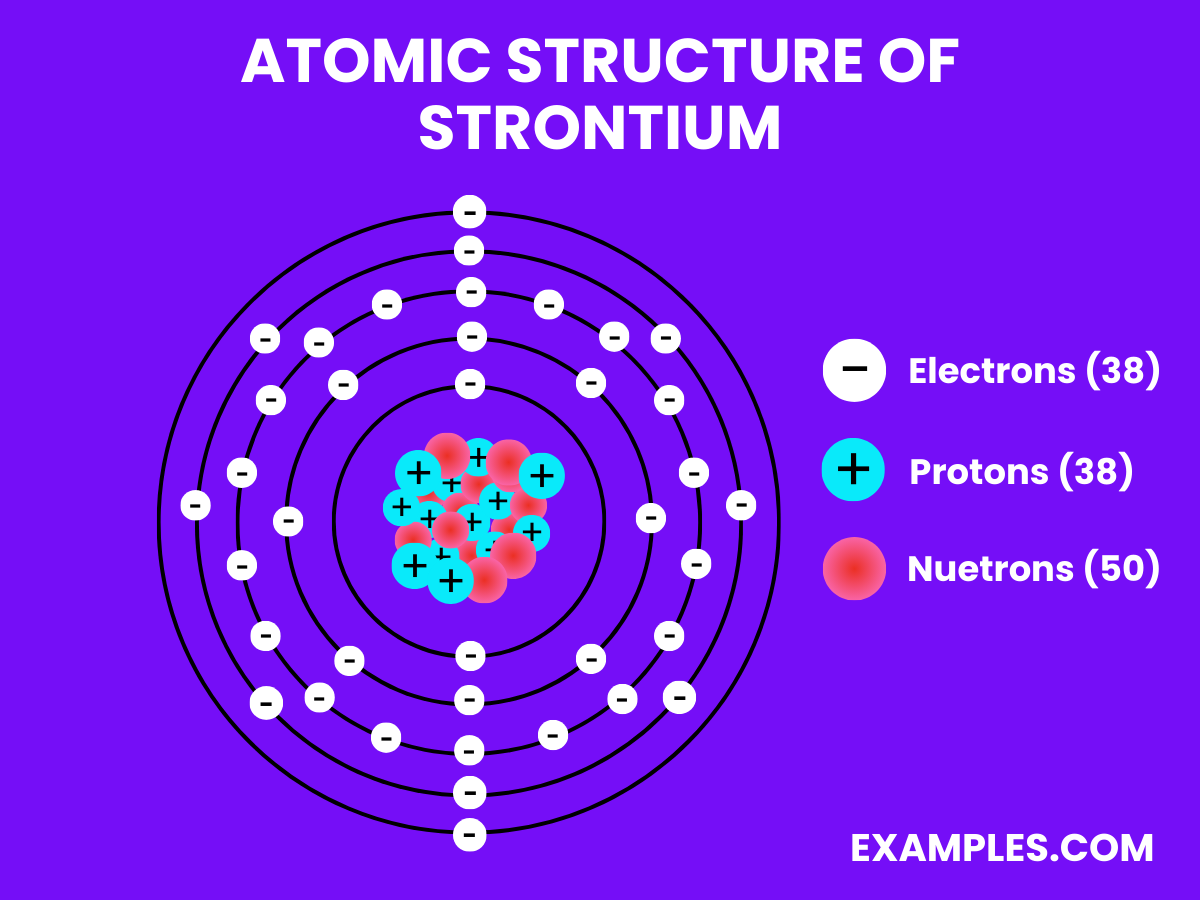
- Electron Configuration: Thirty-eight electrons, with the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s².
- Significance: Used in fireworks for red color, in medical imaging, and in alloys.
- Role in Chemistry: Participates in biochemical processes in the body, similar to calcium.
Atomic Structure of Strontium
Properties of Strontium
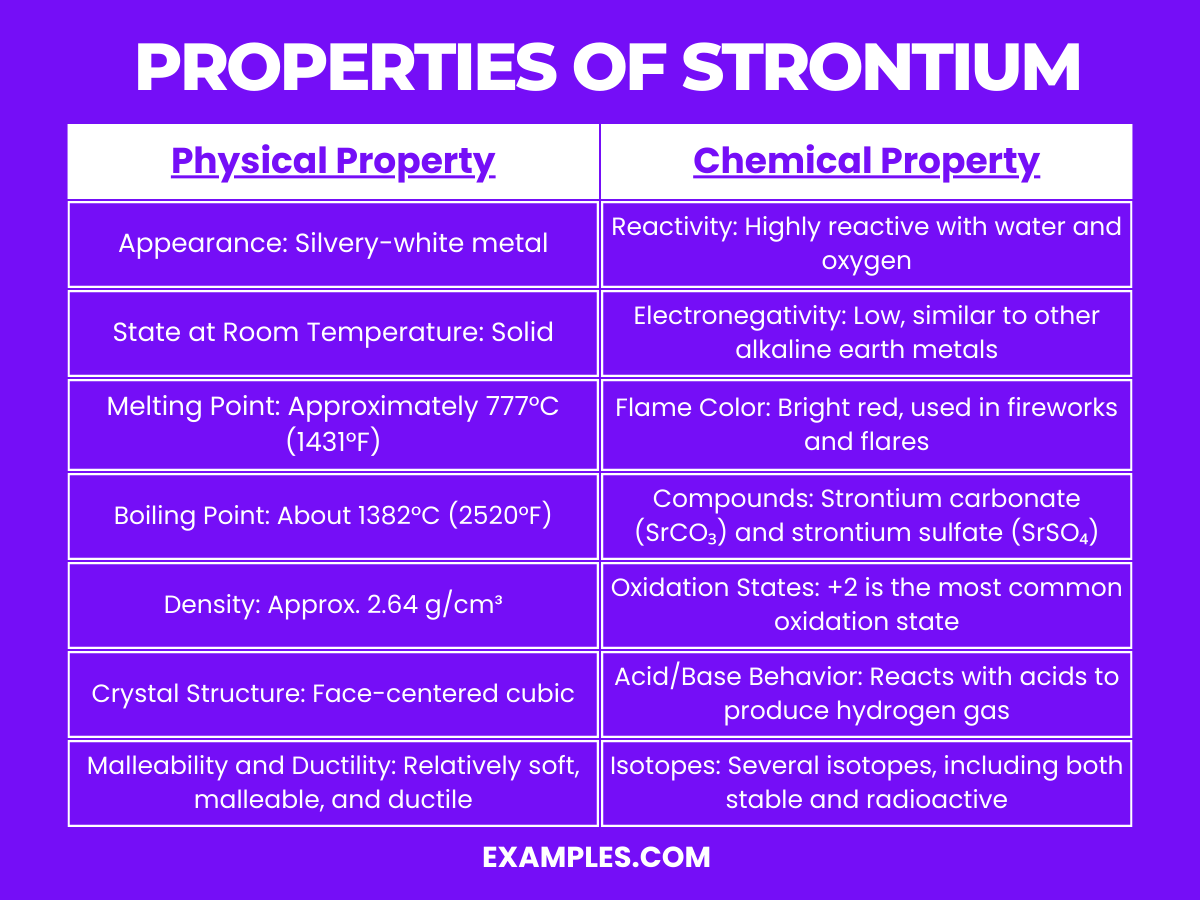
Physical Properties of Strontium
| Property | Description |
|---|---|
| Appearance | Soft, silvery metal that tarnishes in air |
| Melting Point | 777°C |
| Boiling Point | 1382°C |
| Density | 2.64 g/cm³ |
| State at Room Temp. | Solid |
| Malleability | Highly malleable and ductile |
| Conductivity | Good electrical and thermal conductivity |
| Crystal Structure | Face-centered cubic (FCC) |
Chemical Properties of Strontium
Strontium is a reactive element, particularly known for its reactivity with water and air:
- Reactivity with Water: Strontium reacts with water more vigorously than calcium. This reaction is exothermic and releases hydrogen gas, forming strontium hydroxide.
- Reactivity with Air: In air, strontium tarnishes to form a yellowish or pinkish oxide layer. This is due to its reaction with oxygen, which forms strontium oxide.
- Compound Formation: Strontium forms a variety of compounds, including strontium carbonate, strontium sulfate, and strontium nitrate. These compounds are typically ionic in nature.
- Ionization: It easily loses its two outermost electrons to form Sr²⁺ ions, which is indicative of its high reactivity and tendency to form ionic bonds.
- Alloying: Strontium can form alloys with other metals like aluminum for specific applications in manufacturing and industry.
- Isotopes: Strontium has four stable isotopes and several radioactive isotopes. The radioactive isotope Strontium-90, a byproduct of nuclear fission, is of particular interest due to its use in radioisotope thermoelectric generators and its environmental impact.
- Role in Biological Systems: While not heavily involved in biological processes, strontium can influence bone metabolism and is sometimes used in treatments for osteoporosis.
- Flame Test: Strontium imparts a bright red color in a flame test, a characteristic used in pyrotechnics and in identifying strontium compounds.
Thermodynamic Properties of Strontium
| Property | Description / Value |
|---|---|
| Melting Point | 777°C (1431°F) |
| Boiling Point | 1382°C (2520°F) |
| Thermal Conductivity | 35.4 W/(m·K) |
| Specific Heat | 0.300 J/(g·K) |
| Heat of Vaporization | 137.4 kJ/mol |
| Heat of Fusion | 9.2 kJ/mol |
Material Properties of Strontium
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 2.64 g/cm³ |
| Young’s Modulus | 15.7 GPa |
| Tensile Strength | 15-20 MPa |
| Mohs Hardness | 1.5 |
| Elastic Modulus | 15.7 GPa |
Electromagnetic Properties of Strontium
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Paramagnetic |
| Electrical Conductivity | 7.4 MS/m |
Nuclear Properties of Strontium
| Property | Description / Value |
|---|---|
| Atomic Number | 38 |
| Atomic Mass | 87.62 u |
| Neutron Cross Section | 1.28 barns (for ^88Sr) |
| Isotopes | Natural strontium is a mix of ^84Sr, ^86Sr, ^87Sr, and ^88Sr |
| Radioactivity | ^90Sr is a notable radioactive isotope with a half-life of 28.8 years, resulting from nuclear fallout |
Chemical Compounds of Strontium
- Strontium Carbonate (SrCO₃)
- Equation: Sr²⁺ + CO₃²⁻ → SrCO₃
- Used in pyrotechnics for red flames, ceramics, and glass making.
- Strontium Chloride (SrCl₂)
- Equation: Sr²⁺ + 2Cl⁻ → SrCl₂
- Applied in metal refining, and as a dental desensitizer.
- Strontium Sulfate (SrSO₄)
- Equation: Sr²⁺ + SO₄²⁻ → SrSO₄
- Found naturally as the mineral celestine, used in drilling fluids.
- Strontium Nitrate (Sr(NO₃)₂)
- Equation: Sr²⁺ + 2NO₃⁻ → Sr(NO₃)₂
- Common in pyrotechnics for red coloration and in flares.
- Strontium Oxide (SrO)
- Equation: Sr²⁺ + O²⁻ → SrO
- Utilized in ceramics and to prepare other strontium compounds.
- Strontium Hydroxide (Sr(OH)₂)
- Equation: Sr²⁺ + 2OH⁻ → Sr(OH)₂
- Used in refining sugar and as a stabilizer in plastics.
Isotopes of Strontium
| Isotope | Description |
|---|---|
| Strontium-84 | Rare and radioactive, used for scientific research. |
| Strontium-86 | Stable isotope, used in geological studies. |
| Strontium-87 | Stable isotope, plays a crucial role in radiometric dating. |
| Strontium-88 | Most abundant and stable isotope, comprising about 83% of natural strontium. |
| Strontium-89 | Radioactive, used in medicine for treating bone cancer. |
| Strontium-90 | Radioactive, a byproduct of nuclear fission with environmental and health implications. |
Uses of Strontium
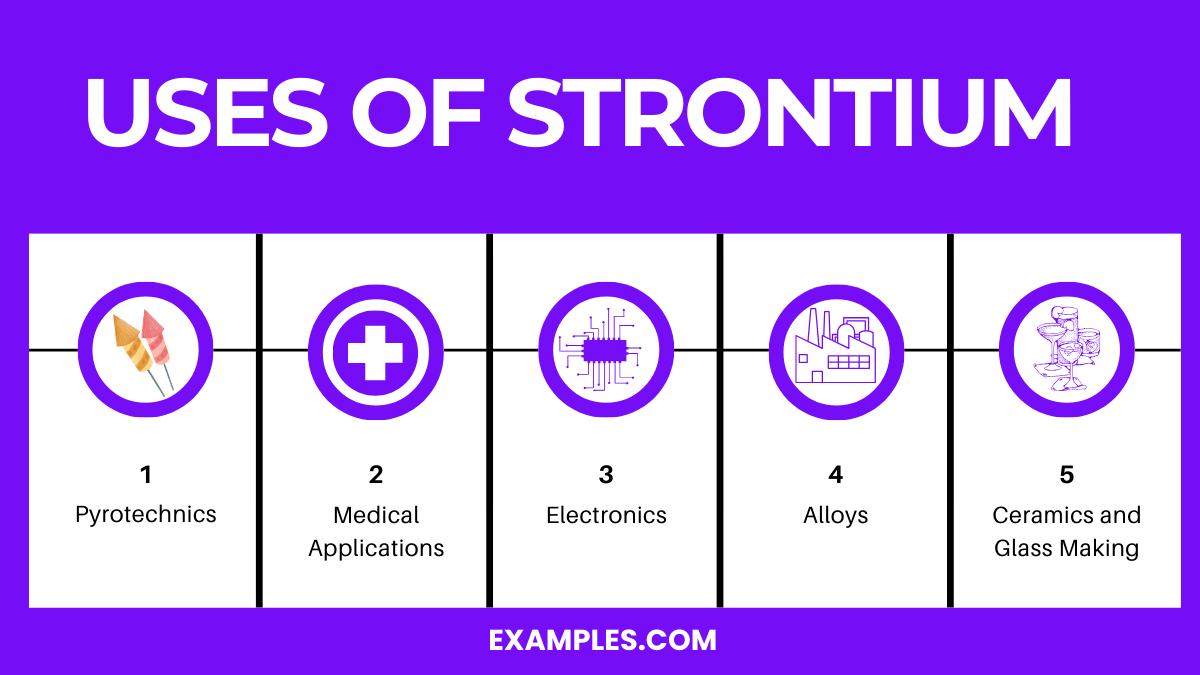
- Pyrotechnics:
- Strontium salts, especially strontium nitrate, are used to impart a deep red color to fireworks and flares. This vibrant color is a hallmark of many pyrotechnic displays.
- Medical Applications:
- Radioactive strontium-89 is utilized in the treatment of bone cancer. It targets bone tissues, providing pain relief and slowing cancer growth in bones.
- Alloys:
- Strontium is added to various alloys to improve their properties. It enhances castability and machinability, particularly in aluminum alloys used in aerospace and automotive industries.
- Electronics:
- Strontium titanate is used in the electronics industry for capacitors and nonlinear resistors, owing to its excellent dielectric properties and high refractive index.
- Ceramics and Glass Making:
- Strontium oxide is used in ceramic magnets and in glass for color television tubes to block X-ray emissions. It also improves the quality and shine in certain types of glass.
Commercial Production of Strontium
The production of strontium predominantly involves the processing of the minerals celestite (strontium sulfate, SrSO₄) and strontianite (strontium carbonate, SrCO₃). Here’s a detailed overview of the process:
- Extraction:
- Strontium is extracted from its ores—celestite and strontianite. These ores are mined from natural deposits.
- Conversion to Carbonate:
- If using celestite, it’s often converted to strontium carbonate since it’s more useful in further applications. This conversion involves treating celestite with sodium carbonate.
- Reduction to Strontium Oxide:
- Strontium carbonate is then reduced to strontium oxide (SrO) in a kiln at high temperatures.
- Electrolytic Reduction:
- Strontium oxide can be further reduced to elemental strontium through electrolysis, but this is less common due to higher costs.
- Refining:
- The produced strontium is refined to achieve the desired purity levels, depending on its intended use.
- Production of Compounds:
- Strontium is often used in the form of compounds. Depending on the application, it may be converted into various chemicals like strontium nitrate for pyrotechnics or strontium chloride for other uses.
Health Effects of Strontium
Strontium’s health effects largely depend on its forms – natural, stable isotopes versus radioactive ones:
Positive Health Effects:
- Bone Health: Natural strontium, especially in the form of strontium ranelate, is sometimes used in osteoporosis treatments. It can mimic calcium, helping to strengthen bone density.
- Dental Health: Some dental products contain strontium chloride, which can help in reducing tooth sensitivity.
Negative Health Effects:
- Radioactive Strontium: Radioactive isotopes like strontium-90 are hazardous. They can be incorporated into bones and bone marrow, potentially causing bone cancer and leukemia.
- Overexposure Risks: Excessive intake of strontium (even in non-radioactive forms) might disrupt calcium absorption, leading to bone health issues and imbalances in body chemistry.
It’s important to distinguish between the health impacts of natural strontium, which can be beneficial, and radioactive strontium, which poses significant health risks.
Environmental Effects of Strontium
The environmental impact of strontium varies depending on its form:
- Natural Strontium:
- As a naturally occurring element, strontium in its stable form is generally harmless to the environment. It is a part of the natural soil and water systems and plays a role in the geological and biological processes.
- Radioactive Isotopes:
- The major concern lies with radioactive isotopes like strontium-90, produced in nuclear reactors and during nuclear explosions. These isotopes can contaminate the environment.
- Strontium-90, due to its long half-life and similarity to calcium, can be absorbed by plants and animals, entering the food chain. It poses a significant risk to wildlife and human health when released into ecosystems, particularly affecting bone health and causing diseases.
- Industrial Usage:
- Industrial activities involving strontium, such as mining and processing, can lead to localized environmental impacts. Proper management and disposal of strontium-containing waste are essential to minimize these impacts.
What Strontium is Used For?
Strontium is used in pyrotechnics, ceramics, medical treatments for bone diseases, alloys in manufacturing, and in the electronics industry.
Is Strontium Harmful to Humans?
Natural strontium is not harmful and can benefit bone health. However, radioactive strontium, like strontium-90, is hazardous and can cause serious health issues.
Why is Strontium Hazardous?
Radioactive strontium is hazardous as it mimics calcium, accumulating in bones and potentially causing bone cancer and leukemia.
What are 5 Facts about Strontium?
- Strontium colors fireworks red.
- It has 16 known isotopes.
- Strontium-90 is a byproduct of nuclear reactions.
- Used in treating osteoporosis.
- Naturally abundant in the earth’s crust.
Strontium’s role in bone health and osteoporosis treatment is significant. Its similarity to calcium allows it to strengthen bone density. However, understanding its use and potential risks is essential for safe and effective application in health and industry.