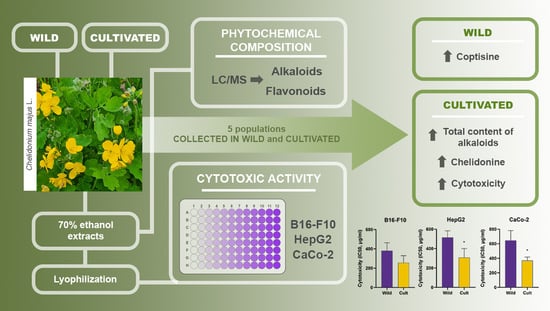
The Cultivation of Chelidonium majus L. Increased the Total Alkaloid Content and Cytotoxic Activity Compared with Those of Wild-Grown Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Alkaloid Profile and Quantitative Analysis of Aqueous Ethanol C. majus Extracts
2.2. Flavonoid Profile and Quantitative Analysis of Aqueous Ethanol C. majus Extracts
2.3. Cytotoxic Activity of Extracts from C. majus
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials and Preparation of Aqueous Ethanol Extracts
3.3. Preparation of Lyophilised Extracts
3.4. HRMS Analysis
3.5. UPLC–MS/MS Analysis of Alkaloids
3.6. UPLC–MS/MS Analysis of Flavonoids
3.7. Cell Culture
3.8. Cytotoxicity Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zielinska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater Celandine’s Ups and Downs-21 Centuries of Medicinal Uses of Chelidonium Majus from the Viewpoint of Today’s Pharmacology. Front. Pharmacol. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priedītis, N. Latvijas Augi [Plants in Latvia]; Riga, Gandrs, 2014; p. 888. [Google Scholar]
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium Majus—An Integrative Review: Traditional Knowledge versus Modern Findings. Forsch. Komplementarmed. 2010, 17, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.J. Chelidonium Majus L.- A Review on Pharmacological Activities and Clinical Effects. J. Med. Plants. 2013, 2, 238–245. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.; Phillipson, D. The monograph: Celandine, Greater. In Herbal Medicines: A Guide for Healthcare; Pharmaceutical Press: London, UK, 2007; pp. 136–145. [Google Scholar]
- Sile, I.; Romane, E.; Reinsone, S.; Maurina, B.; Tirzite, D.; Dambrova, M. Data on Medicinal Plants in the Records of Latvian Folk Medicine from the 19th Century. J. Ethnopharmacol. 2020, 28, 105024. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Klepacki, P.; Łuczaj, Ł. Fischer’s Plants in Folk Beliefs and Customs: A Previously Unknown Contribution to the Ethnobotany of the Polish-Lithuanian-Belarusian Borderland. J. Ethnobiol. Ethnomed. 2017, 13, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menković, N.; Šavikin, K.; Tasić, S.; Zdunić, G.; Stešević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical Study on Traditional Uses of Wild Medicinal Plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Fortini, P.; di Marzio, P.; Guarrera, P.M.; Iorizzi, M. Ethnobotanical Study on the Medicinal Plants in the Mainarde Mountains (Central-Southern Apennine, Italy). J. Ethnopharmacol. 2016, 184, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Pranskuniene, Z.; Dauliute, R.; Pranskunas, A.; Bernatoniene, J. Ethnopharmaceutical knowledge in Samogitia region of Lithuania: Where old traditions overlap with modern medicine. J. Ethnobiol. Ethnomed. 2018, 14, 1–26. [Google Scholar] [CrossRef]
- European Medicines Agency. Public Statement on Chelidonium Majus L., Herba. Available online: https://Www.Ema.Europa.Eu/En/Documents/Public-Statement/Final-Public-Statement-Chelidonium-Majus-l-Herba_en.Pdf (accessed on 30 July 2021).
- Och, A.; Zalewski, D.; Komsta, Ł.; Kołodziej, P.; Kocki, J.; Bogucka-Kocka, A. Cytotoxic and Proapoptotic Activity of Sanguinarine, Berberine, and Extracts of Chelidonium Majus L. and Berberis Thunbergii DC. toward Hematopoietic Cancer Cell Lines. Toxins 2019, 11, 485. [Google Scholar] [CrossRef] [Green Version]
- Deljanin, M.; Nikolic, M.; Baskic, D.; Todorovic, D.; Djurdjevic, P.; Zaric, M.; Stankovic, M.; Todorovic, M.; Avramovic, D.; Popovic, S. Chelidonium Majus Crude Extract Inhibits Migration and Induces Cell Cycle Arrest and Apoptosis in Tumor Cell Lines. J. Ethnopharmacol. 2016, 190, 362–371. [Google Scholar] [CrossRef]
- Rica Capistrano, I.; Wouters, A.; Lardon, F.; Gravekamp, C.; Apers, S.; Pieters, L. In Vitro and in Vivo Investigations on the Antitumour Activity of Chelidonium Majus. Phytomed 2015, 22, 1279–1287. [Google Scholar] [CrossRef]
- Ernst, E.; Schmidt, K. Ukrain—A New Cancer Cure? A Systematic Review of Randomised Clinical Trials. BMC Cancer 2005, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Grosso, C.; Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Sampaio, M.; Lima, J.; Andrade, P.B. Box-Behnken Factorial Design to Obtain a Phenolic-Rich Extract from the Aerial Parts of Chelidonium Majus L. Talanta 2014, 130, 128–136. [Google Scholar] [CrossRef]
- Maji, A.K.; Banerji, P. Chelidonium Majus L. (Greater Celandine)—A Review on Its Phytochemical and Therapeutic Perspectives. Int. J. Herb. Med. 2015, 3, 10–27. [Google Scholar] [CrossRef]
- Seidler-Łożykowska, K.; Kędzia, B.; Bocianowski, J.; Gryszczyńska, A.; Łowicki, Z.; Opala, B.; Pietrowiak, A. Content of Alkaloids and Flavonoids in Celandine (Chelidonium Majus L.) Herb at the Selected Developmental Phases. Acta Sci. Pol. Hortorum Cultus. 2016, 15, 161–172. [Google Scholar]
- Wianowska, D.; Garbaczewska, S.; Cieniecka–Roslonkiewicz, A.; Typek, R.; Dawidowicz, A.L. Chemical Composition and Antifungal Activity of Chelidonium Majus Extracts–Antagonistic Action of Chelerythrine and Sanguinarine against Botrytis Cinerea. Chem. Ecol. 2018, 34, 582–594. [Google Scholar] [CrossRef]
- Jesionek, W.; Fornal, E.; Majer-Dziedzic, B.; Móricz, Á.M.; Nowicky, W.; Choma, I.M. Investigation of the Composition and Antibacterial Activity of UkrainTM Drug Using Liquid Chromatography Techniques. J. Chromatogr. A 2016, 1429, 340–347. [Google Scholar] [CrossRef]
- Sárközi, Á.; Janicsák, G.; Kursinszki, L.; Kéry, Á. Alkaloid Composition of Chelidonium Majus L. Studied by Different Chromatographic Techniques. Chromatographia 2006, 63, 81–86. [Google Scholar] [CrossRef]
- Zielinska, S.; Wójciak-Kosior, M.; Dziagwa-Becker, M.; Glensk, M.; Sowa, I.; Fijalkowski, K.; Ruranska-Smutnicka, D.; Matkowski, A.; Junka, A. The Activity of Isoquinoline Alkaloids and Extracts from Chelidonium Majus against Pathogenic Bacteria and Candida Sp. Toxins 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Zielínska, S.; Czerwínska, M.E.; Dziagwa-Becker, M.; Drys, A.; Kucharski, M.; Jezierska-Domaradzka, A.; Płachno, B.J.; Matkowski, A. Modulatory Effect of Chelidonium Majus Extract and Its Alkaloids on LPS-Stimulated Cytokine Secretion in Human Neutrophils. Molecules 2020, 25, 842. [Google Scholar] [CrossRef] [Green Version]
- Jakovljević, Z.D.; Stanković, S.M.; Topuzović, D.M. Seasonal Variability of Chelidonium Majus L. Secondary Metabolites Content and Antioxidant Activity. EXCLI J. 2013, 12, 260–268. [Google Scholar] [CrossRef]
- Qing, Z.X.; Cheng, P.; Liu, X.b.; Liu, Y.S.; Zeng, J.G. Systematic Identification of Alkaloids in Macleaya Microcarpa Fruits by Liquid Chromatography Tandem Mass Spectrometry Combined with the Isoquinoline Alkaloids Biosynthetic Pathway. J. Pharm. Biomed. Anal. 2015, 103, 26–34. [Google Scholar] [CrossRef]
- Orvos, P.; Virág, L.; Tálosi, L.; Hajdú, Z.; Csupor, D.; Jedlinszki, N.; Szél, T.; Varró, A.; Hohmann, J. Effects of Chelidonium Majus Extracts and Major Alkaloids on HERG Potassium Channels and on Dog Cardiac Action Potential—A Safety Approach. Fitoterapia 2015, 100, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomè, F.; Colombo, M.L. Distribution of Alkaloids in Chelidonium Majus and Factors Affecting Their Accumulation. Phytochemistry 1995, 40, 37–39. [Google Scholar] [CrossRef]
- Samatadze, T.E.; Yurkevich, O.Y.; Hazieva, F.M.; Konyaeva, E.A.; Morozov, A.I.; Zoshchuk, S.A.; Amosova, A.V.; Muravenko, O.V. Agro-Morphological, Microanatomical and Molecular Cytogenetic Characterization of the Medicinal Plant Chelidonium Majus L. Plants 2020, 9, 1396. [Google Scholar] [CrossRef] [PubMed]
- Parvu, M.; Vlase, L.; Fodorpataki, L.; Parvu, O.; Rosca-Casian, O.; Bartha, C.; Barbu-Tudoran, L.; Parvu, A.E. Chemical Composition of Celandine (Chelidonium Majus L.) Extract and Its Effects on Botrytis Tulipae (Lib.) Lind Fungus and the Tulip. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.Z.; Chen, P.; Ozcan, M.; Harnly, J.M. Chromatographic Profiles and Identification of New Phenolic Components of Ginkgo Biloba Leaves and Selected Products. J. Agric. Food Chem. 2008, 56, 6671–6679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-readi, M.Z.; Eid, S.; Lotfy, M.; Tahrani, A.; Wink, M. Modulation of Multidrug Resistance in Cancer Cells by Chelidonine and Chelidonium Majus Alkaloids. Phytomedicine 2013, 20, 282–294. [Google Scholar] [CrossRef]
- Fadhil, Y.B.; Alsammarraie, K.W.; Mohaimen, N.A.; Mohammed, Z.Y. In Vitro Cytotoxic Activity of Chelidonium Majus Extract Using Different Types of Cell Lines. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1767–1775. [Google Scholar] [CrossRef]
- Petruczynik, A.; Tuzimski, T.; Plech, T.; Misiurek, J.; Szalast, K. Comparison of Anticancer Activity and HPLC-DAD Determination of Selected Isoquinoline Alkaloids. Molecules 2019, 24, 3417. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]

| RT, min | m/z | Compound | MW (Monoisotopic) | Calculated Elemental Composition |
|---|---|---|---|---|
| 18.02 | 354.134 | Protopine 1 | 353.126 | C20H19NO5 |
| 18.81 | 354.133 | Chelidonine | 353.126 | C20H19NO5 |
| 19.00 | 370.163 | Allocryptopine 1 | 369.158 | C21H23NO5 |
| 19.09 | 320.092 | Coptisine 1 | 320.092 | C19H14NO4+ |
| 20.2 | 370.165 | Allocryptopine 1 | 369.158 | C21H23NO5 |
| 20.33 | 340.118 | Norchelidonine 1 | 339.111 | C19H17NO5 |
| 20.56 | 340.153 | Canadine 1 | 339.147 | C20H21NO4 |
| 21.05 | 340.118 | Norchelidonine 1 | 339.111 | C19H17NO5 |
| 21.28 | 332.091 | Sanguinarine | 332.092 | C20H14NO4+ |
| 21.87 | 336.122 | Berberine 1 | 336.124 | C20H18NO4+ |
| 23.46 2 | 348.122 | Chelerythrine | 348.124 | C21H18NO4+ |
| 23.46 2 | 382.128 | 6,10-Dihydroxyl chelerythrine 3 | 382.129 | C21H20NO6+ |
| Compound | Average Alkaloid Content (n = 5) | p Value | |
|---|---|---|---|
| Wild 2019 | Cultivated 2020 | ||
| Sanguinarine | 1.9 ± 2.1 | 12.8 ± 3.6 | 0.0004 |
| Chelerythrine | 3.5 ± 1.3 | 17.5 ± 8.5 | 0.007 |
| Chelidonine | 63.6 ± 35.4 | 252.2 ± 133.2 | 0.02 |
| Coptisine 1 | 138.5 ± 35.6 | 143.5 ± 32.2 | 0.8 |
| Berberine 1 | 9.4 ± 6.6 | 12.8 ± 8.4 | 0.6 |
| Allocryptopine 1 | 5.2 ± 3.0 | 11.9 ± 7.4 | 0.1 |
| Total Content | 222.0 | 450.6 | 0.02 |
| Peak # | RT, min | Characteristic ions 1 ESI+, m/z | Characteristic ions 1 ESI−, m/z | Compound | MW (Monoisotopic) | Calculated Elemental Composition 2 | Parent Scan of Aglycone Fragment Ion, m/z |
|---|---|---|---|---|---|---|---|
| 1 | 11.2 | 627.144 | Quercetin Triglycoside | 772.206 | C33H40O21 | 773, 627,465 (303) 3 | |
| 2 | 13.0 | 755.201 | Kaempferol Triglycoside | 756.211 | C33H40O20 | 757, 611, 449 (287) | |
| 3 | 13.6 | 479.119 | Isorhamnetin Triglycoside | 786.222 | C34H42O21 | 787, 641, 479 (317) | |
| 4 | 16.8 | 611.159 | 609.143 | Quercetin 3-O-Rutinoside | 610.153 | C27H30O16 | 611, 465 (303) |
| 5 | 17.0 | 465.101 | Quercetin 3-O-Galactoside | 464.100 | C21H20O12 | 465 (303) | |
| 6 | 17.4 | 287.056 | 593.147 | Kaempferol Diglycoside | 594.158 | C27H30O15 | 595, 449 (303) |
| 7 | 18.4 | 287.054 | 593.147 | Kaempferol 3-O-Rutinoside | 594.101 | C27H30O15 | 595, 449 (287) |
| 8 | 18.8 | 449.104 | 447.089 | Kaempferol Glucoside | 448.101 | C21H20O11 | 449 (287) |
| 9 | 19.0 | 625.173 | 623.158 | Isorhamnetin 3-O-Rutinoside | 624.169 | C28H32O16 | 626, 279 (317) |
| 10 | 19.4 | 479.119 | 477.102 | Isorhamnetin Glycoside | 478.111 | C22H22O12 | 479 (317) |
| Compound | Average Flavonoid Content (n = 5) | p Value | |
|---|---|---|---|
| Wild 2019 | Cultivated 2020 | ||
| Kaempferol | 13.1 ± 9.2 | 6.9 ± 4.2 | 0.2 |
| Isorhamnetin | 8.8 ± 6.7 | 4.0 ± 1.6 | 0.2 |
| Quercitrin | 1.4 ± 1.2 | 2.0 ± 0.9 | 0.4 |
| Isorhamnetin 3-O-Rutinoside | 1612.7 ± 722.9 | 1857.2 ± 326.0 | 0.5 |
| Kaempferol 3-O-Rutinoside | 653.8 ± 377.4 | 600.7 ± 216.4 | 0.8 |
| Quercetin 3-O-Rutinoside | 3007.2 ± 1270.1 | 4385.1 ± 1150.8 | 0.1 |
| Quercetin 3-O-Galactoside | 220.2 ± 269.9 | 195.9 ± 114.0 | 0.9 |
| Kaempferol Glucoside 1 | 135.9 ± 130.8 | 53.5 ± 12.9 | 0.2 |
| Total | 5653.1 | 7105.3 | 0.3 |
| Sample | IC50 (µg/mL) ± SD | ||
|---|---|---|---|
| HepG2 | B16-F10 | CaCo-2 | |
| Wild 2019 | |||
| CHM01 | 422.67 ± 1.09 | 264.85 ± 1.13 | >500 |
| CHM02 | >500 | 354.81 ± 1.22 | >500 |
| CHM03 | >500 | 496.59 ± 1.05 | >500 |
| CHM04 | >500 | 389.94 ± 1.12 | >500 |
| CHM05 | 461.32 ± 1.13 | 394.46 ± 1.08 | >500 |
| Cultivated 2020 | |||
| CHM01 | 351.56 ± 1.38 | 279.25 ± 1.08 | 361.41 ± 1.84 |
| CHM02 | 241.55 ± 1.22 | 174.98 ± 1.12 | 291.07 ± 1.10 |
| CHM03 | 272.27 ± 1.16 | 325.84 ± 1.20 | 406.44 ± 1.08 |
| CHM04 | 226.46 ± 1.66 | 318.42 ± 1.08 | 389.94 ± 1.20 |
| CHM05 | 448.75 ± 1.34 | 180.30 ± 1.54 | 400.87 ± 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krizhanovska, V.; Sile, I.; Kronberga, A.; Nakurte, I.; Mezaka, I.; Dambrova, M.; Pugovics, O.; Grinberga, S. The Cultivation of Chelidonium majus L. Increased the Total Alkaloid Content and Cytotoxic Activity Compared with Those of Wild-Grown Plants. Plants 2021, 10, 1971. https://doi.org/10.3390/plants10091971
Krizhanovska V, Sile I, Kronberga A, Nakurte I, Mezaka I, Dambrova M, Pugovics O, Grinberga S. The Cultivation of Chelidonium majus L. Increased the Total Alkaloid Content and Cytotoxic Activity Compared with Those of Wild-Grown Plants. Plants. 2021; 10(9):1971. https://doi.org/10.3390/plants10091971
Chicago/Turabian StyleKrizhanovska, Valerija, Inga Sile, Arta Kronberga, Ilva Nakurte, Ieva Mezaka, Maija Dambrova, Osvalds Pugovics, and Solveiga Grinberga. 2021. "The Cultivation of Chelidonium majus L. Increased the Total Alkaloid Content and Cytotoxic Activity Compared with Those of Wild-Grown Plants" Plants 10, no. 9: 1971. https://doi.org/10.3390/plants10091971