ANTIVIRAL ACTIVITY OF EXTRACT OF CACTUS
TECHNICAL FIELD
This invention relates to antimicrobial properties identified in cactus plants in particular Opuntia streptacantha; the antiviral properties comprises inhibition of replication of viruses in cells and secondly inactivation of extracellular infectious virus particles. There is inhibition of
intracellular virus replication when the virus-infected cells are incubated with extract of cactus in the incubation medium but also by pre-incubation of the cells in extract-containing medium with removal of this medium prior to virus infection of the cells. The field of this invention extends to both DNA and RNA viruses and is operative in human and non-human cell lines; in addition the field extends to treatment of human and
non-human species towards prevention or treatment of virus infections.
BACKGROUND ART
A number of plant extracts have been shown to have antiviral effects in terms of inhibiting the replication of viruses in invitro assay systems. As examples, inhibition of replication of herpes simplex virus type 1 by Geranium sanguincum LVIII, influenza, herpes simplex and human immunodeficiency virus by pine cone antitumour substance, murine cytomegalovirus by
Chlorella vulgaris and poliovirus by Ulex europaeus have been reported (Zgorniak-Nowosielska et al 1989, Nagata et al 1990, Ibusuki et al 1990, De Rodriguez et al 1990, Sakagami et al 1989). In addition, a number of substances from plants are capable of neutralising the infectivity of viruses invitro;
some of these substances are lectins, for example Concanavalin A from Conovalia ensiformis will neutralise infectivity of herpes simplex virus, human cytomegalovirus, Epstein-Barr virus and human immunodeficiency disease virus (Ito and Barron 1974, Ito et al 1978, Khelifa and Meneces 1982, Lifson et al 1986).
Plant extracts have been shown to inhibit replication of other non-viral micro-organisms, namely bacteria and mycobacteria
(Macfoy et al 1990, Grange et al 1990) and protozoa for example malaria and trypanosomes (Ghandi et al 1990, Igweh et al 1989). There is urgent need for effective non-toxic and inexpensive products to prevent, control or treat virus infections of vertebrates, non-vertebrates and plant species. In human subjects, available medications for virus infections are extremely limited and present drugs tend to be expensive or toxic; Acyclovir (Zovirax) is a useful parenteral, oral and topical medication for herpes simplex virus and
varicella-zoster virus infections but is expensive and many patients are not prescribed this drug by their medical
practitioners on account of cost particularly in the United Kingdom where there has been increasing pressure and
restriction on family practitioner budgets within recent years. Gancyclovir for the treatment of cytomegalovirus infections and Zudovadine for human immunodeficiency virus infections (Aids) are both expensive and highly toxic drugs and only offer limited promise in the therapy of these increasingly important infections.
A second problem with certain presently available antiviral drugs is that they tend to operate on only virus-infected cells; this is most notable with Acyclovir where the molecule acycloguanosine is phosphorylated to the monophosphate by the virus-coded enzyme thymidine kinase with only a low level of phosphorylation by uninfected cells and only at high
concentrations of the drug. Thus the inhibitory effect of these drugs depends defacto on virus macromolecular events in the cell to initiate its operative mechanism. There is thus less prospect for such drugs in terms of prevention of virus replication at both the cellular level and the therapeutic level in a multicellular host.
Cactus plants and extracts of said plants have been used for decorative, nutritional and medicinal purposes. As an example, extracts of Opuntia Streptacantha have been used for some years as oral hypoglycaemic agents in the control of diabetes - and of special interest to the subject matter of this specification - a number of plants support virus growth without detriment or even with benefit to the plant; alternatively, virus infection
can result in pathological damage or death to the plant (Delay 1969; Koenig 1972; Boiko et al 1972; Nelson and Tremaine 1975).
It is therefore surprising that cactus extracts will inhibit the intracellular replication of viruses and will inactivate extracellular viruses.
DISCLOSURE OF INVENTION
This invention tea.ches that pre-incubation or incubation of cells with extracts of cactus plants will reduce replication of DNA and RNA viruses and at appropriate concentrations will inhibit synthesis of any new infectious virus particles; the extract will also inactivate extracellular virus.
In the experiments to be described in this disclosure, the dried powder from one capsule was suspended in 5mls of growth medium usually Eagles modified medium containing 10% calf serum and 10% tryptose phosphate broth for 15 minutes at 37° C
centrifuged at 800g for 10 minutes, the supernatant
recentrifuged under the same conditions and this second
supernatant used as stock solution; this solution usually contained aproximately 60mgm protein per ml. The extract was routinely filtered through 0.45/ym Millipore filters before use. Stock solutions were normally freshly prepared for
experimentations.
The following experiments are presented to indicate salient features of the invention by way of example.
1. Inhibition of replication by incubation of HSV-2
virus-infected cells in medium containing cactus extract
Monolayers of baby hamster kidney cells (BHK-21) were infected with 1 plaque forming unit (pfu) per cell of HSV2 ; following 1 hour absorption at 37°C, the monolayers were washed twice with medium and appropriate concentrations in 3mls of medium of a centrifuged filtered preparation of Opuntia streptacantha added to the monolayers. One monolayer was put to -70°C to provide estimate of input level of virus, namely the amount of virus
absorbed to the monolayers at time zero prior to incubation and virus replication. Following incubation for 24 hours at 37°C in a gassed (CO2) incubator, the medium was removed and
intracellular virus titrated following disruption of the cells by ultrasonic vibration; virus was titrated by the suspension plaque assay method of (Russel et al (1962)).
The results indicate that the extract will reduce virus
replication over 10 fold at a concentration of 3.5mg/ml and will reduce replication to input levels (where there is no virus replication) at a concentration of 15mg/ml of incubation medium. There is therefore evidence of inhibition of virus replication by addition of this compound to the growth medium. Data for other viruses is indicated in Table 1; there was significant inhibition of replication of DNA and RNA viruses including the retrovirus human immunodeficiency virus (HIV). It was also found that replication of HSV2 in organ culture explants of human cervix was inhibited by 3.5 Log10 following addition of 15mgm per ml of extract to the incubation medium. This indicates that the active components of the extract will penetrate and operate in virus-infected contiguous whole tissue which more closely resembles in-vivo conditions than obtain in cell culture.
2. Inhibition of virus replication by pre-incubation of cells in cactus containing medium a. Type 2 herpes simplex virus; 24hr pre-incubation
Monolayers of BHK baby hamster kidney cells (cell line BHK21 Mcpherson & Stoker 1962) were prepared by additon of 4 X 10 cells to 5cm plastic petri dishes and allowed to incubate overnight to form a sub-confluent monolayer. The medium was removed and replaced by medium containing the following
concentration of Streptacantha opuntia namely 1.8mg per ml of medium to 15mgs per ml in two-fold dilution steps and the cells allowed to incubate in the cactus containing medium for a further 24 hours. The cactus-containing medium and
cactus-free medium on control monolayers were removed and the cells washed twice with fresh cactus-free medium followed by addition of 10 plaque forming units of type 2 herpes simplex
virus strain in 2ml of medium. The virus was allowed to absorb for 1 hour at 37° C after which it was removed and 4ml of fresh medium added. The virus was allowed to replicate within the cells for a further 24 hours after which the supernatant medium was harvested and stored separately from the virus infected cells which were removed from the Petri dish by a rubber policeman and then resuspended in 1ml of water and stored at -70°C.
Both the infected cells and the supernatant were thawed, the infected cells disrupted by ultrasonic vibration and the sample titrated by suspension plaque assay by the method of Russell (1962).
The results are indicated in Table 2. There was a significant reduction in replication of intracellular virus which
correlated with increased concentrations of extract in the medium. There was also a reduction in the titre of
extracellular virus as measured in the supernatant medium
(Table 2). b. Type 2 herpes simplex; influenza A virus. 48hr
pre-incubation
An identical experiment with 48hr pre-incubation using type 2 herpes simples and influenza virus (influenza A NWS strain) was carried out (Table 3). There was a similar reduction in replication of both viruses with no replication in medium with a concentration of more than 3.8mgs per ml. Comparison of the reduction in virus titre for type 2 herpes simplex virus in cells with only 24hr pre-incubation with cactus extract
indicated little difference - a favourable finding suggesting that prolonged pre-incubation of cells in the extract may not be necessary for a significant reduction in virus replication. Similar results were obtained using a simple water-extraction preparation from a fresh cactus plant; 30g of fresh cactus which had been transported from Mexico to the U.K. were dried in 10ml of sterile water, crushed and incubated at 25°C for 3hrs. The liquid portion was withdrawn subjected to ultrasonic vibration of 4 minutes in a Megason water bath sonicator; this constituted the stock solution from which appropriate dilutions
were made in Eagles's medium with 10% calf serum and 10% tryptose phosphate broth.
As it is known that certain constituents of plants - for example lectins - are capable of virus neutralisation, a series of experiments where BHK21 cells were treated with cactus extract were examined as cell extracts for neutralising
activity against type 2 herpes simplex virus. There was no evidence of virus neutralisation by the cell extract which therefore did not contribute to the aforesaid reduction in intracellular virus replication.
3. Specificity of virus inhibition by extract
The effect of cactus extract on synthesis of virus (HSV2) polypeptides and uninfected cell polypeptides was investigated by examining the uptake of 35 S-methionine into trichloro-acetic acid precipitable polypeptides which were then identified by autoradiography (Table 4). While important virus polypeptides were not evident at 15mg/ml and were reduced in intensity at 7.5mg/ml of extract in medium, there was no loss of uninfected cell polypeptides at 15mg of extract per ml of medium.
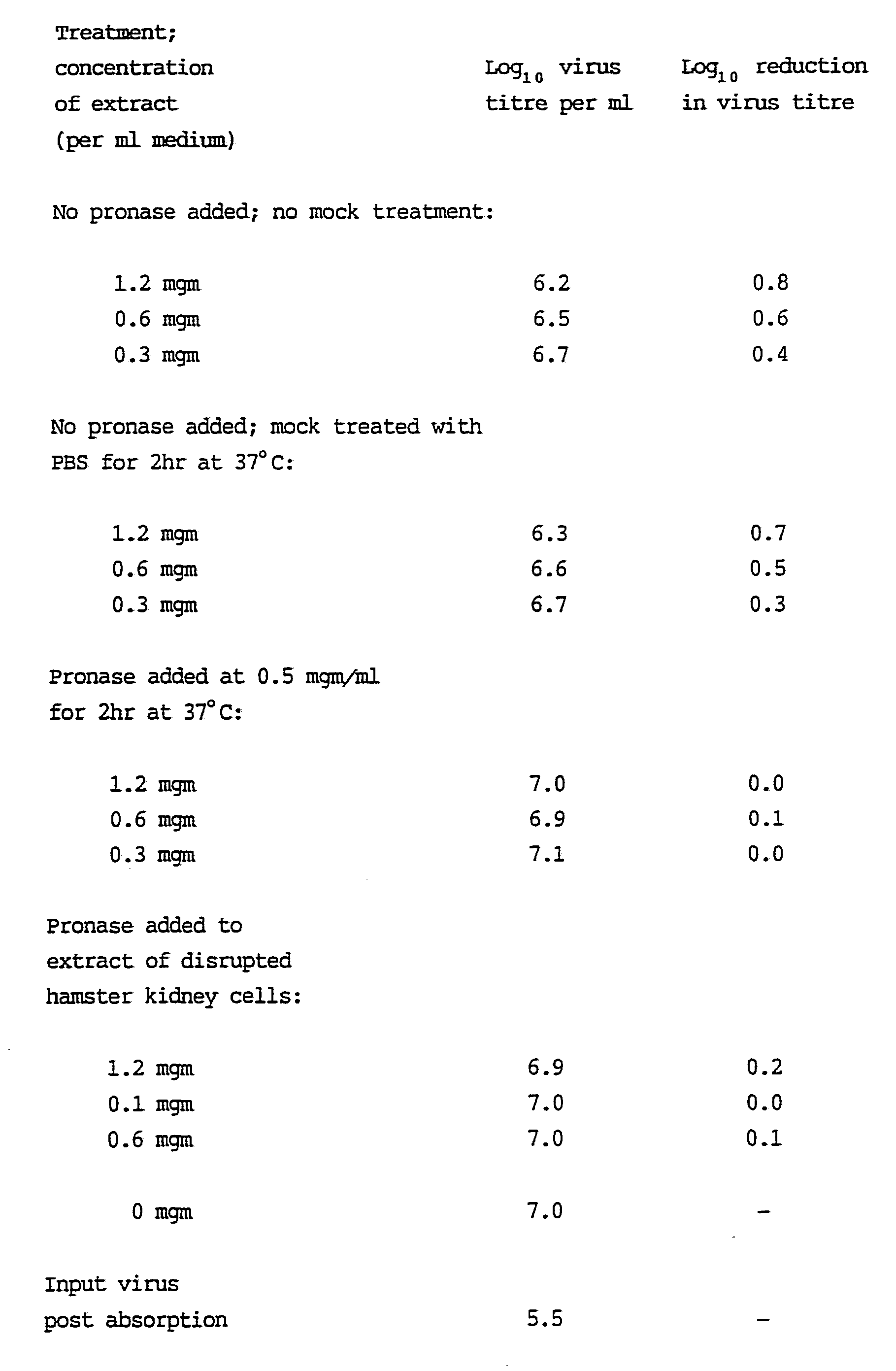
4. Evidence that active components of the extract are likely to be protein in nature
Prior to addition of extract to medium the extract was
pre-treated with pronase under conditions indicated in Table 5 and with trypsin under conditions where the extract was added prior and following virus infection (Tables 6a and 6b
respectively). There was a reduction in the virus inhibitory effect of the extract which related (approximately) to
increasing concentration of the proteolytic enzyme in the extract. In addition, liquid phase extraction with ether and chloroform did not reduce viral inhibitory activity while precipitation with acetone and reconstitution to the same concentration did not effect the virus inhibitory activity
(Table 7). Finally, there was no significant reduction in virus inhibitory activity of the extract following dialysis. Therefore, it seems likely that the active components were
protein in nature and not members of the alkaloid, flavanoid or tripterpene groups which are found in various types of plants including cacti and are also known to have antiviral
properties.
5. Location of inhibitory activity to wall of plant
The different parts of the cactus plant, namely cuticle wall and inner sap were extracted and tested at the concentrations as given in Table 8; it should be noted that these
concentrations do not correspond with other concentrations in this disclosure as these are obtained from the fresh plant and are not concentrated dried powders. It is clear that the virus inhibitory activity resided in the wall of the plant and not the cuticle or inner sap.
6. Inactivation of extracellular virus (HSV2) by cactus
extract
Extracellular cell-free HSV2 was incubated at 37° C for 1 and 24 hours with varying concentrations of cactus extract in Eagles medium; virus was also incubated with medium alone (cactus free) as a control (Table 9). There was significant reduction in titre of HSV2 at both 1 and 24 hours.
Thus the cactus extract inhibits replication of virus within cells and in addition neutralises or inactivates extracellular virus.
7. Evidence that virus inhibitory levels of active components are present in human sera following ingestion of cactus extract
A patient ingested eight capsules per day (2.4gms per day) in divided doses of 300mg in each capsule for five days; a blood sample was drawn prior to ingestion of the capsules and four hours following ingestion of the last capsule. The serum was separated off and added to medium in BHK21 cells infected with herpes simplex virus type 2 (HSV2) or equine herpes virus I (EHV1); virus yields from these cells were compared to yields
from virus-infected cells incubated in cactus free medium
(Table 10).
Replication of HSV2 was (as expected) reduced in the presence of both pre-ingestion and post-ingestion serum; this was explicable on account of high HSV2 neutralising antibody levels in this serum operating either by reducing the spread of virus from cell to cell or by inhibition of virus release from virus infected cells. However there was a significantly higher level of inhibition of virus replication with the serum removed following ingestion of the cactus extract indicating an
additional inhibitory effect over the pre-ingestion serum.
Towards examining if this effect would be obtained with a herpes virus of animal species namely equine herpes virus I (EHVl) and, perhaps more importantly to use a virus wherein there are no neutralising antibodies in human sera (thus excluding the complication which obtained with HSV2) the test was repeated with this virus in the same experimental system. There was insignificant reduction in virus replication with the pre-ingestion serum while in the serum removed following ingestion of the extract there was a significant reduction (0.66 Log10) indicating that this serum had acquired a level of active components from ingestion of the extracxt that inhibited the replication of EHV1 in this laboratory test system.
8. Clinical efficacy of extract
The cactus extract prepared from Opuntia streptacantha has been tested in patients with troublesome recurrent herpes genitalis towards modification of the frequency and severity of
recurrences (Table 11 ). Patients were given four to six capsules per day as indicated in Table 11. The pattern of disease following medication was compared to the pattern prior to medication which was obtained in a prospective fashion in anticipation of instituting treatment with the plant extract in three months time once three months information on the pattern of the disease had been established. Patients were interviewed and given a questionnaire at two months following institution of medication and in addition asked to give an opinion as to whether the treatment had improved the pattern of disease, made
it worse or had not changed the pattern at all. The results of this preliminary investigation are indicated in Table 11; every patient had evidence of improvement in terms of pattern of disease and 17 of the 20 patients (85%) reported improvement. It is acknowledged that this study was neither
placebo-controlled nor double-blinded but serves to indicate an encouraging effect on this clinical assessment of a limited number of patients; in my opinion I have found this extract to be the most encouranging form of treatment during 20 years experience of this condition which has included use of
Acylovier, Immunovir and other proprietory preparations
designed to modify the pattern of this disease.
The extract has been used at six capsules per day in 6 patients with an acute episode of herpes labialis and in 8 patients during an acute episode of herpes genitalis. There was a decrease in duration of herpetic lesions and extent of
vasiculation when the course of the episode was compared to the usual pattern of disease (when extract was not used) although it is acknowledged that the latter information is
retrospective.
The extract was used as a local applicant at a concentration of 100mg per ml in sterile saline to five patients with episodes of oral herpes labialis; patients were asked to apply the lotion eight times per day and particularly before retiring to sleep at night. Every patient reported benefit in terms of reduced healing time and less discomfort following regular application of the lotion during the five days of treatment. The formulation of the lotion for herpes labialis or other facial herpetic eruptions may be modified in the future as the brown colour and its slightly earthy taste was not particularly pleasing to two of the female patients although this did not result in cessation of treatment.
In summary there is evidence of benefit from the extract in terms of modification of the pattern of recurrent herpes genitalis and treatment of acute episodes either by oral ingestion or as a local medicament in the form of a lotion.
There have been no side effects from either oral ingestion of local application of the extract.
REFERENCES
Zgorniak-Nowosielska, I. et al. Acta Microbiol. Bulg. 1989, 24 3-8.
Nagata, K. et al. Antiviral Res. 1990, 13 (1) 11-21.
Ibusuki, E. et al. Nat. Immunol. Cell Growth Regnt. 1990, 9 (2) 121-128.
De Rodriguez, et al. Planta Med. 1990, 56 (1) 59-62.
Sakagami, H. et al. Anticancer Res. 1989, 9 (6) 1593-8.
Ito, M. and Barron, A.L. J. Virol. 1974, 13 (6) 1312-1318.
Ito, M. Girin, L. and .Barron A.L. Arch. Virol. 1978, 57
97-105.
Khelifa, R. and Meneces. J. Virol. 1982, 42 (2) 402-410.
Lifson, J. et al . J . Exp . Medicine . 1986 , 14 2101-2106 .
Macfoy, C. et al. J. Ethnopharmacol. 1990, 28 323-328.
Grange, J.M. J. Applied Bacteriol. 1990, 68 587-591.
Ghandi, M. et al. J. Ethnopharmacol. 1990, 29 51-57.
Igweh, A.C. et al. Anπu Trep. Med. Parasitol. 1989, 83
527-534.
Delay, C. Acad. Sci. (D) (Paris) 1969, 269 (16) 1510-1513.
Koenig, R. Virology 1972, 50 (1) 263-266.
Boiko, A.L. et al. Mikrobiol. Zh. 1972, 34 523-525.
Nelson, M.R. and Tremaine, J.H. Virology 1975, 65 (2) 309-19.
Russel, W.C. Nature. 1962, 195 1028-1029
MacPherson, I. and Stoker, M. Virology 1962, 16 147.
Table 1. Inhibition of replication of DNA and RNA. viruses.
Extract was used at a concentration of 3.5mg/ml. Cytomegalovirusa had higher inhibition of replication on post-infection than pre-infection treatment of cells; this probably relates to the longer incubation times of this virus (3-4 days) and consequently longer exposure to the components of the extract.
Table 2. Effect of pre-incubation of cells for 24hr by cactus extract on replication of type 2 herpes simplex virus
Table 4. Inhibition of HSV2 but not host cell polypeptide synthesis; labelling at 1hr - 16hr of infection.
These differences were detected by protein anlaysis and by autoradiographic detection of synthesised 3 5 S-methionine labelled polypeptides ( Figure I ) .
Table 5. Effect of pronase on inhibitory activity of extract
Table 6a. Effect of trypsin on inhibitory activity of extract; pre-infection treatment of cells
Table 6b. Effect of trypsin on inhibitory activity of extract post-infection treatment of cells
Table 7. Effect of lipid solvents on inhibitory activity of extract
Table 8. Distribution of inhibitory activity in extract from different parts of the plant leaf
Table 9. Inactivation of extracellular virus ( HSV2 )
Virus and extract were reacted at 37°C in Eagle's medium containing 10% calf serum and 10% tryptose phosphate broth. The reaction was stopped by dilution into cold medium which also reduced the concentration of extract to below <0.07mgm per ml at which there would not be inhibition of virus replication by the extract.
Table 10. Inhibition of replication of type 2 herpes simplex (HSV2) and equine herpes virus I (EHV1) by serum from a patient ingesting the extract.
Serum was at 25% concentration in medium for HSV2-infected cells and at 40% concentration for EHV-1 infected cells.