WO2011042409A2 - Aqueous emulsions of polyorganosiloxanes - Google Patents
Aqueous emulsions of polyorganosiloxanes Download PDFInfo
- Publication number
- WO2011042409A2 WO2011042409A2 PCT/EP2010/064786 EP2010064786W WO2011042409A2 WO 2011042409 A2 WO2011042409 A2 WO 2011042409A2 EP 2010064786 W EP2010064786 W EP 2010064786W WO 2011042409 A2 WO2011042409 A2 WO 2011042409A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- groups
- group
- aqueous emulsions
- emulsion
- polyorganosiloxanes
- Prior art date
Links
- 0 CC1C(C)**C1 Chemical compound CC1C(C)**C1 0.000 description 5
- XJODJHDMUSLQAU-UHFFFAOYSA-N CC(C)C(CCC1(C)C)CC1O Chemical compound CC(C)C(CCC1(C)C)CC1O XJODJHDMUSLQAU-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L83/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers
- C08L83/04—Polysiloxanes
- C08L83/06—Polysiloxanes containing silicon bound to oxygen-containing groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/04—Dispersions; Emulsions
- A61K8/06—Emulsions
- A61K8/068—Microemulsions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/60—Sugars; Derivatives thereof
- A61K8/604—Alkylpolyglycosides; Derivatives thereof, e.g. esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/89—Polysiloxanes
- A61K8/896—Polysiloxanes containing atoms other than silicon, carbon, oxygen and hydrogen, e.g. dimethicone copolyol phosphate
- A61K8/898—Polysiloxanes containing atoms other than silicon, carbon, oxygen and hydrogen, e.g. dimethicone copolyol phosphate containing nitrogen, e.g. amodimethicone, trimethyl silyl amodimethicone or dimethicone propyl PG-betaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/02—Preparations for cleaning the hair
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/12—Preparations containing hair conditioners
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L83/00—Compositions of macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing silicon with or without sulfur, nitrogen, oxygen or carbon only; Compositions of derivatives of such polymers
- C08L83/04—Polysiloxanes
- C08L83/08—Polysiloxanes containing silicon bound to organic groups containing atoms other than carbon, hydrogen and oxygen
Definitions
- This instant invention pertains to clear aqueous microemulsions of polyorganosiloxanes containing polar groups and alkylpolyglycosides.
- the emulsions of this invention are free of polyglycols and surfactants with oxyalkylene functional groups.
- Aqueous emulsions of polyorganosiloxanes are well known. Clear microemulsions of polysiloxanes are less common and their preparation is limited to specific polyorganosiloxanes with polar pendant groups and specific limiting combinations of surfactants and specific process steps, see US 4, 146,499, US 4,620,878, US 5,057,572, US 5,244,598, US 5,258,451 , US 5,302,657, US 5,683,625, US 6, 147,038, US 6,451 ,905, and US 6,547,490.
- the microemulsion compositions described all use organic ethoxylates as surfactants and do not describe the use of alkylpolyglycosides (APGs).
- Aqueous emulsions of polyorganosiloxanes are used in many applications, for example, as release agents, slip agents, water repellants, in the treatment of textiles and other fibrous materials and in detergents, cosmetic and other derma tological formulations for personal care.
- the use of emulsions of polyorganosiloxanes containing polar pendant groups in personal care formulations is well documented, for example, in US 4,529,586, US 4,563,347, US 4,559,227, US 4,563,347 and US 4,586518.
- Personal Care formulations are also known (US 5,035,832, US 5,409,628, DE 4,229,922 and US 5,690,920) which claim the use of both silicones and alkylpolyglycosides, but in none of these cases do the alkylpolyglycosides serve the purpose of emulsifying the polysiloxane and the polysiloxane is not claimed to be a microemulsion.
- the use of clear aqueous microemulsions of polyorganosiloxanes in personal care formulations have been described in US 6, 153,569 and US 6, 147,038, but these documents do not describe the use of alkylpolyglycosides to emulsify the polysiloxane.
- the US 5,466,746 describes the use of alkylpolyglycosides to make emulsions with the composition: a) 100 parts by weight of polyorganosiloxanes which contain polar groups on Si-C-bonded hydrocarbon radicals, with the proviso that methyl or phenyl groups are not to be included with the Si-C-bonded hydrocarbon radicals which contain polar groups, and
- the claimed cosurfactants are C 4 -C 8 -alcohols, glycol ethers, esters or ketones. These compositions do not spontaneously give rise to microemulsions, but as a rule require the use of high shear forces. Microemulsions are also only obtained in the presence of one of the claimed cosurfactants.
- the DE 41 31 551 describes the use of alkylpolyglycosides to obtain aqueous dispersions of amino- or amido substituted polyorganosiloxanes for the treatment of fabrics.
- the compositions comprise:
- At least one of the surfactants in b) is an alkylpolyglycoside.
- Possible cosurfactants are defined as fatty alcohol ethoxylates and the like.
- Preferred dispersions contain only alkylpolyglycoside surfactants.
- Microemulsions are obtained by acidification of the emulsion according to WO 98/008436. The stability of these dispersions towards neutralization and/or electrolytes is not described. It has been observed that the preferred microemulsions comprising only alkylpolyglycosides do not exhibit the desired stability towards neutralization or electrolytes.
- microemulsions of polyorganosiloxanes containing polar pendant groups could not be obtained according to the method of DE 4131551 with alkylpolyglycosides as the only surfactants, if the content of polar pendant groups is low.
- High contents of polar pendant groups are commonly to be avoided as these groups can easily be oxidized resulting in coloration (yellowing) in the end use product.
- polyorganosiloxanes a2) with less than 0.9 mmol/g polar pendant groups, whereas this concentration relates to polydimethylsiloxanes and has to be adjusted accordingly the molweight of other polyorganosiloxanes where methyl groups are replaced by other organo groups. It is especially desirable to be able to make microemulsions of polyorganosiloxanes with between 0.3 and 0.9 mmol/g polar pendant groups.
- Microemulsions of silicones a2) with polar pendant groups are particularly desirable raw materials in the preparation of personal care formulations for hair and skin care. These microemulsions are commonly formulated with additional surfactants, other oil phases and water. The resulting formulation is as a rule buffered to a neutral pH and may contain added electrolytes as thickening agents. A key requirement of these microemulsions is that they have to be stable to dilution with water, pH shifts and/or added electrolytes.
- the objective of the instant invention is to provide optically clear microemulsions of polyorganosiloxanes containing polar groups, for example, pendant polar groups, which can be made with a minimum of shear energy and which are stable to dilution with water, neutralization and addition of electrolytes.
- the instant invention provides clear microemulsions that are free of polyglycols, organic ethoxylates and other surfactants with oxyalkylene functional groups.
- ionic and non-ionic cosurfactants that do not contain alkylene oxide groups do not give stable microemulsions of polyorganosiloxanes containing polar pendant groups.
- the list of cosurfactants that do not give stable microemulsions includes but is not limited to sorbitan and glycerin esters of fatty acids, and anionic surfactants such as alkyl sarcosinates and their salts, alkyl isethionates and their salts, as well as alkyl sulfonates and their salts. Without being bound by theory it is believed that the positive charge or dipole in the surfactant structure is required to obtain stable microemulsions of polyorganosiloxanes, in particular, those containing polar pendant groups.
- microemulsions form spontaneously by simple mixing and the properties of the emulsions are independent of the order of addition, the pH of the emulsion or the shear force used in their preparation.
- These microemulsions are extraordinarily stable, remaining unchanged by storage at elevated temperatures or shifts in pH from acid to neutral or basic.
- the microemulsions are also stable to dilution with water and addition of electrolytes such as sodium chloride.
- the instant invention describes aqueous, finely divided, optically clear oil-in-water silicone emulsions comprising: a) 100 parts by weight of one or more polyorganosiloxanes which contain one or more polar groups on Si-C-bonded hydrocarbon radicals, b) > 50 - 150 parts by weight of one or more alkylpolyglucosides, c) 0.1 - 150 parts by weight of one or more co-surfactants selected from the group consisting of amphoteric or zwitterionic surfactants, amine oxide surfactants and cationic surfactants, d) water, preferably a) 100 parts by weight of one or more polyorganosiloxanes which contain one or more polar groups on Si-C-bonded hydrocarbon radicals, b) > 50 - 150 parts by weight of one or more alkylpolyglucosides, c) 0.1 - 150 parts by weight of one or more co-surfactants selected from the group consisting of amphoteric or zwitterio
- Component a) is preferably a polyorganosiloxane, in which the polar groups bonded to Si-C-bonded hydrocarbon radicals are selected from the group consisting of amino, ammonium, epoxy, hydroxyl, amido, mercapto, carboxyl, carboxylic acid ester, sulphonic acid, sulphonic acid ester groups or salts thereof, preferably from amino and ammonium groups.
- Preferred polar groups are amino groups and/or ammonium groups, including:
- Monofunctional polar groups -NH 2 , -NH 3 + , -NHR, -NH 2 R + , -NHR 2 + , -NR 2 , -NR 3 + , wherein R is an organic group, in particular, an optionally substituted C -C 22 -alkyl group, wherein one or more methylene groups are replaced by -NH-, -0-, -CO-, - NR-.
- Substitutents of the Ci-C 2 2-alkyl group include in particular 1 to 3 groups selected from hydroxy and -NH 2 , These monofunctional amino or ammonium groups are preferably used in polyorganosiloxanes a2) that have pendant polar groups, attached to silicone atoms of a polydiorganosiloxane moiety via a hydrocarbon residue.
- polyorganosiloxanes a2) that have pendant polar groups are described more specifically below.
- Di- or polyfunctional polar groups including groups like -NH-, - H 2 + -, -NR-, - NHR + -, -NR 2 + -, wherein R is an organic group, are in particular used in the polyamino- and/or polyammonium-polysiloxane compounds a1 ) as described in the following.
- the polar group is in general part of the molecule backbone formed of polydiorganosiloxane moieties and polar group-containing moieties, which are attached to the polydiorganosiloxane moieties via hydrocarbon residues, comprising a SiC-bond.
- Component a) includes, in particular, polyamino- and/or polyammonium-polysiloxane compounds a1 ) and/or polar polysiloxane compounds a2) having pendant polar groups preferably amino- and/or ammonium groups.
- the polyamino- and/or polyammonium-polysiloxane compound a1 ) is a copolymer compound, which has amino and/or ammonium repeat units and polysiloxane repeat units in the polymer main chain.
- the amino units contain secondary and/or tertiary nitrogen atoms (2 or 3 organic radicals on the uncharged nitrogen atom).
- the ammonium units contain secondary, tertiary and/or quaternary, positively charged nitrogen atoms (2, 3 or 4 organic radicals on the nitrogen).
- the amino and/or ammonium repeat units may also serve heterocyclic radicals bonded into the polymer chain via two nitrogen atoms.
- polysiloxane compounds a2) have pending polar groups as defined above.
- these polar groups are pendant amino- and/or ammonium groups in polysiloxane compounds showing the structure a2) below.
- the compounds a2) contain polar groups preferably amino and/or ammonium groups in the pendant groups of the polysiloxane main chain.
- the pendant groups are linked over Si-C bonds to the Si atoms. In other words, the amino and/or ammonium groups are not present in the main chain comprising the polysiloxane repeat units.
- components a1 ) and a2) may be present alone or together.
- component a2) is, however, present alone in the inventive formulation, without component a1 ).
- both component a1 ) and component a2) are present together.
- Components a1 ) or a2) may be present together in any ratios relative to one another.
- the polyamino- and/or polyammonium-polysiloxane compound a1 ) preferably comprises polysiloxane compounds which contain at least one unit of the formula (I):
- R is in each case hydrogen or a monovalent organic radical, where Q is not bonded to a carbonyl carbon atom,
- V is at least one constituent which is selected from the group consisting of V 1 , V 2 and V 3 , where V 2 is selected from divalent, straight chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radicals which have up to 1000 carbon atoms (not counting the carbon atoms of the polysiloxane radical Z 2 defined below) and may optionally contain one or more groups selected from
- R 2 is hydrogen, a monovalent, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 100 carbon atoms, may contain one or more groups selected from -0-, -NH-, -C(O)- and -C(S)-, and may optionally be substituted by one or more substituents selected from the group consisting of a hydroxy I group, an optionally substituted heterocyclic group preferably containing one or more nitrogen atoms, amino, alkylamino, dialkylamino, ammonium, polyether radicals and polyether ester radicals, where, when a plurality of - CONR 2 groups is present, they may be the same or different,
- the V 2 radical may optionally be substituted by one or more hydroxy I groups, and the V 2 radical contains at least one -Z 2 - group of the formula
- V 1 is selected from divalent, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radicals which have up to 1000 carbon atoms and may optionally contain one or more groups selected from
- R 2 is as defined above, where the R 2 groups in the V 1 and V 2 groups may be the same or different,
- R 1 is as defined above, where the R 1 groups in the V 1 and V 2 groups may be the same or different, and
- n 2 from 0 to 19
- V 1 radical may optionally be substituted by one or more hydroxyl groups
- V 3 is a trivalent or higher-valency, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 000 carbon atoms, may optionally contain one or more groups selected from
- Z 3 is a trivalent or higher-valency polyorganosiloxane unit, and may optionally be substituted by one or more hydroxy I groups, where, in said polysiloxane compound, in each case one or more V 1 groups, one or more V 2 groups and/or one or more V 3 groups may be present, with the proviso
- said polysiloxane compound contains at least one V 1 , V 2 or V 3 group which contains at least one -Z 1 -, -Z 2 - or Z 3 group, and
- the tri- and tetravalent Q radicals either serve to branch the main chain formed from Q and V, so that the valencies which do not serve for bonding in the main chain bear further branches formed from -[Q-V]-units, or the tri- and tetravalent Q radicals are saturated with V 3 radicals within a linear main chain without formation of a branch, and wherein the positive charges resulting from ammonium groups are neutralized by organic or inorganic acid anions, and acid addition salts thereof.
- the polysiloxane compounds which contain at least one unit of the formula (I) are terminated by monofunctional -Q-R and/or -V-R groups, i.e., for example, by amino groups. These arise by saturation of one of the two binding points of Q or V by a monovalent R group or hydrogen, which is as defined above, and are also referred to below as V st or Q st . Instead of V st , other unconverted reactive groups such as epoxy or haloalkyl groups may also be present.
- polysiloxane compounds which contain at least one unit of the formula (I) are also intended to include the case where only one -[Q-V]- unit is present, so that compounds of the formula R-V-[Q-V]-R or R-[Q-V]-Q-R, where R may also be replaced by H, are also included.
- Suitable polyamino- and/or polyammonium-polysiloxane compounds a1 are described, for example, in WO 02/10257, WO 02/10259, DE-A 100 36 522, DE-A 100 36 532, DE-A 100 36 533 and the unpublished DE application 102 12 470.1 .
- the compounds may also be according to US 6,240,929.
- the polyorganosiloxane compounds that contain at least one unit of the formula (I) are, for example, linear polysiloxane copolymers of the general formula ( ⁇ ):
- V is at least one V 1 group and at least one V 2 group,
- V 1 and V 2 are each as defined above.
- V may also be trivalent or higher-valency, particularly trivalent, V 3 radicals.
- tri- or tetravalent Q units as defined above, are also present, and the trivalent or higher-valency V 3 radicals and the tri- or tetravalent Q units are saturated exclusively by one another within the linear main chain to form cyclic structures, as illustrated in more detail below. However, this case is less preferred.
- Q is therefore selected from the group consisting of:
- V is selected from V 1 and V 2 .
- the molar ratio of the V 1 and V 2 groups in the polysiloxane compounds V 2 /V 1 may in principle assume any value.
- the invention thus also includes the case in which the polysiloxane compound of the formulae (I) or ( ⁇ ) contains only V 2 units, i.e. the polysiloxane compound has the formula -[Q-V 2 ]-.
- the invention also embraces the case in which the polysiloxane compound contains only V 1 units. However, in this case, the V 1 units have to contain Z 1 -siloxane units.
- the polysiloxane compound of the formulae (I) or ( ⁇ ) contains both V 2 and V 1 units.
- the molar ratio of the V 1 and V 2 groups in the polysiloxane compounds of the general formulae (I) and ( ⁇ ) is:
- V 2 /V 1 1
- Such linear amine and tetraorganoammonium compounds have been described, for example, in WO 02/10257, WO 02/10259, EP 282720 or US 5,981 ,681.
- Particular preference is given to the polysiloxanes of WO 02/10259 and WO 02/10257, and reference is made here explicitly to the polysiloxane polymers defined in claim 1 , which form part of the disclosure of the present application.
- V 2 /V 1 does not equal 1 ; preferably, V 2 /V 1 is ⁇ 1 , more preferably ⁇ 0.9; even more preferably, V 2 /V 1 fulfills the relationship
- the R group is preferably selected from the R 2 groups.
- Q is a divalent radi is selected in the formulae (I) or ( ⁇ ) from the group consisting of:
- R is as defined above, preferably R 2 , R 2 is as defined above, and the definition of R 2 and the definition of the above R 2 group may be the same or different, R 3 has the definition of R 2 , where R 2 and R 3 may be the same or different, or R 2 and R 3 together with the positively charged nitrogen atom form a five- to seven-membered heterocycle, which may optionally have one or more nitrogen, oxygen and/or sulfur atoms in addition,
- R 5 , R 6 , R 7 may be the same or different and are selected from the group consisting of: H, halogen, hydroxy I group, nitro group, cyano group, thiol group, carboxyl group, alkyl group, monohydroxyalkyl group, polyhydroxyalkyl group, thioalkyl group, cyanoalkyl group, alkoxy group, acyl group, acetyloxy group, cycloalkyl group, aryl group, alkylaryl group, and groups of the -NHR W type in which R w is H, alkyl group, monohydroxyalkyl group, polyhydroxyalkyl group, acetyl group, ureido group, and in each case two of the adjacent R 5 , R 6 and R 7 radicals with the carbon atoms binding them to the heterocycles may form aromatic five- to seven-membered rings, and
- R 8 has the definition of R 2 , where R 8 and R 2 may be the same or different.
- V 2 is a group of the formula
- V 2* is a divalent, straight-chain cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 40 carbon atoms and may optionally contain one or more groups selected from -0-, -CONH-, -CONR 2 - in which R 2 is as defined above, -C(O)- and -C(S)-, and the V 2* radical may optionally be substituted by one more hydroxyl groups.
- the inventive linear polysiloxane copolymer may have the following repeat units: -[V 2* -Z 2 -V 2* -Q]-, preferably together with -[V 1 -Q]-
- V 2* is a preferably a divalent straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 16 carbon atoms, may be substituted by one or more groups selected from -0-, -CONH-, -CONR 2 - in which R 2 is as defined above, -C(O)-, -C(S)- and may be substituted by one or more hydroxy I groups. Even more preferably, -V 2* - is selected from groups of the formulae:
- -CH CHC3 ⁇ 4OC(0)CH 2 -, -CH-CHCH 2 OC(0)CH 2 CH 2 -,
- R 2 is preferably: H
- R 4 straight-chain, cyclic or branched C- ⁇ to Ci 8 -hydrocarbon radical which may be substituted by one or more groups selected from -0-, -NH-, -C(O)- and -C(S)- and may be substituted by one or more OH groups, especially unsubstituted C 5 to Ci 7 -hydrocarbon radicals which derive from the corresponding fatty acids or else hydroxylated C 3 to Ci7-radicals which can be traced back to hydroxy I ated carboxylic acids, especially saccharide carboxylic acids, and quite especially
- R 2 is preferably: in whic 5 to R 7 are each as defined above,
- V 1 is preferably
- R 9 is a divalent, saturated or mono- or polyunsaturated, straight-chain or branched hydrocarbon radical having from two to 25 carbon atoms,
- u is from 1 to 3
- q and r are each from 0 to 200, preferably from 0 to 100, more preferably from 0 to 70 and particularly preferably from 0 to 40, and
- V 1 Preferred variants of V 1 are structures of the formula:
- r from 0 to 200.
- q from 1 to 50, in particular from 2 to 50, especially from 1 to 20, very especially from 1 to 10, and also 1 or 2
- r from 0 to 100, in particular from 0 to 50, especially from 0 to 20, very especially from 0 to 10, and also 0 or 1 or 2.
- the polysiloxanes a1 may be prepared as described in the patents above.
- the polysiloxanes of the general formula (I) used as component a1 ) in accordance with the invention may also contain branch units V 3 .
- V 3 is a trivalent or higher-valency, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 1000 carbon atoms and may optionally contain one or more groups selected from -0-, -CONH-, -CONR 2 - where R 2 is as defined above, -C(O)-, -C(S)-, -Z 1 -, which is as defined above, -Z 2 - which is as defined above and Z 3 where Z 3 is a trivalent or higher-valency polyorganosiloxane unit.
- the branch unit V 3 may be silicone-free. Examples thereof include:
- the branch unit V 3 may be a trivalent or high-valency polyorganosiloxane unit, for example:
- Z -containing branch unit V 3 is, for example:
- the polyamino- and/or polyammonium-polysiloxane compounds a1 ) used may be solid or liquid at 25 °C.
- They may have melting points up to 250 °C, but are water-soluble or -dispersible. Their solubility is preferably more than 1 g/l at 25°C.
- the preferred concentration of amino or ammonium units are in the range of 2 to 4 amino or ammonium units per 50 -100 organosiloxy units.
- the component a) used may be preferably one or more one amino- and/or ammonium-polysiloxane compounds a2). As explained above, these contain amino or ammonium groups only in the pendant groups.
- the amino- and/or ammonium-polysiloxane compounds a2) are preferably polysiloxanes which bear primary and/or secondary and/or tertiary amino groups in the pendent groups, in which the amino groups may optionally be protonated or quaternized, and which may optionally contain additional hydrophilic groups.
- the amino or ammonium groups mentioned are preferably bonded to the siloxane skeleton via carbon.
- amino- and/or ammonium-polysiloxane compounds a2) mentioned are preferably polyalkylsiloxanes having aminoalkyl- or aminoarylsiloxane units.
- the aminoalkyl units may be bonded to the difunctional, trifunctional or the monofunctional end groups, and be part of other oxygen-containing pendent groups, in particular of polyether pendent groups.
- the additional hydrophilicizing groups optionally present are preferably those, which derive from polyalkylene oxides and saccharides.
- aminopolysiloxanes mentioned are linear or branched polysiloxanes which are formed from siloxy units which are selected from the group consisting of:
- R 11 represents organic radicals which may be the same or different with the proviso that at least one of the R 11 radicals contains at least one nitrogen atom.
- R 11 substituents are preferably selected from the group consisting of:
- R 2 may be hydrogen, a monovalent, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 100 carbon atoms, may contain one or more groups selected from -0-, -NH-, -C(O)- and -C(S)-, and is optionally substituted by one or more substituents selected from the group consisting of a hydroxyl group, an optionally substituted heterocyclic group preferably containing one or more nitrogen atoms, amino, alkylamino, dialkylamino, ammonium, polyether radicals and polyether ester radicals, where, when a plurality of -NR 2 groups is present, they may be the same or different,
- radical may optionally be substituted by one or more substituents selected from hydroxyl and where R 1 , m, m2 are each as defined above, hydroxy I,
- polyether radical which has up to 20 000 carbon atoms and may optionally bear one or more amino, mono- or dialkylamino, or arylamino groups,
- R 1 1 substituents from different siloxy units together form a straight- chain, branched or cyclic alkanediyi radical having from 2 to 12 carbon atoms between two silicon atoms,
- At least one R 1 1 substituent per molecule contains nitrogen, i.e., constitutes a nitrogen-containing R 11 radical.
- R 11 is preferably alkyl, in particular methyl.
- R 1 1 radicals which have nitrogen are, for example:
- amino- and ammonium-containing R 11 radicals are, for example:
- R 1 1 radicals are disclosed in WO 02/10256, whose disclosure content belongs to the present application.
- the nitrogen-containing R 1 1 radical is preferably aminopropyl or aminoethyl- aminopropyl. Further preferred nitrogen-containing R 1 1 radicals are formed from the reaction of glycidyloxypropylsiioxanes with mono- or dialkylamines.
- Preferred compounds as component a2) are therefore, for example, aminopolysiloxanes which arise from the reaction of epoxyalkylsiloxanes with ammonia, primary or secondary amines, such as those from the reaction of glycidyloxypropylsiioxanes with mono- or dialkylamines.
- Preferred alkoxy radicals for R 1 1 are methoxy, ethoxy, propoxy, isopropoxy, butoxy, hexyloxy and cyclohexyloxy.
- Preferred polyether radicals which have up to 20 000 carbon atoms and may optionally bear one or more amino-, mono- or dialkylamino, or arylamino groups for R 1 1 include, for example:
- Saccharide-containing organic radicals for R 1 1 are, for example:
- saccharide-containing polysiloxanes can be found, for example, in DE 4 318 536, DE 4 318 537.
- the average degree of polymerization of the polysiloxane moiety of the aminopolysiloxanes a2) used in accordance with the invention, which results from Mn, is appropriately from 1 to 3000, preferably from 50 to 1000.
- the typical content of polar groups in the polyorganosiloxanes a2) is between 0.03 % by weight to 3 % by weight.
- the preferred polar group in a2) are amino groups, wherein the typical nitrogen content of such aminopolyorgano- siloxanes a2) used in accordance with the invention is, for example, between 0.03 % by weight to 3 % by weight, preferably from 0.3 % by weight to 1.3 % by weight, more preferably from 0.4% by weight to 1.2 % by weight.
- This relates to aminopolyorganosiloxanes a2), wherein polyorganosiloxanes are polydimethyl- siloxanes.
- the values for the nitrogen concentration have to be adjusted for polyorganosiloxanes having other groups than methyl.
- They may have melting points up to 250 °C, but are water-soluble or -dispersible. Their solubility is preferably more than 1 g/l at 25 °C.
- aminopolysiloxanes a2) used in accordance with the invention are obtainable, for example, as Wacker Finish* WR 1 100 and Momentive ® SF 1923.
- compositions according to the invention contain component a2).
- the polyorganosiloxanes containing polar groups of the present invention are preferably any polysiloxanes having at least one Si-C bonded polar organic group selected from the group consisting of amino, ammonium, epoxide, hydroxy, polyhydroxy, mercapto, or carboxyl and sulfonic acids, their esters and salts.
- Preferred are polyorganosiloxanes containing Si-C bound pendant amino or ammonium, hydroxy I or carboxyl acid polar groups.
- E is CrC 22 -alkyl, preferably methyl, aryl, preferably phenyl,
- the component a2) contains at least 0.3 mmol/g of the polar groups, in particular, the amino and/or ammonium groups. Still more preferably the component a2) contains between 0.3 and 0.9 mmol/g of the polar groups, in particular, the amino and/or ammonium groups. Particularly preferred is the range of 0.35 to 0.6 mmol/g in particular of the amino and/or ammonium groups. These concentrations apply to polydimethylsiloxane compounds. The value 0.3 mmol/g corresponds approximately to 2.2 polar groups per 100 dimethylsiloxy groups. These compounds may contain traces of alkoxysilyl groups coming out of an uncompleted condensation reaction of the precursors.
- the composition according to the invention contains more than 50 to 150 parts by weight (pw), preferably >50 to 120 pw of one or more alkylpolyglucosides (sometimes referred to as APG), based on 100 parts of component a). Still more preferably the weight ratio of the alkylpolyglucosides b) to the polyorganosiloxanes a) is less than 1 or the amount of the alkylpolyglucosides b) is less than 100 pw based on 100 pw of component a). Preferably the weight ratio of the APGs to the component a) is between 0.5 to 1.1 , preferably between 0.5 and 1 .0.
- the alkylpolyglucosides b) are preferably at least one compound selected from the group of the following formula (II)
- AO-[G] p (II) wherein the unit ' G ' is a substituted or non-substituted, preferably non- substituted sugar unit, respectively, containing preferably 5 or 6 carbon atoms and the index " p ' is a number of 1 to 10, and ' A ' is preferably selected from the group of straight- or branched-chain C 4 - C 24 -alkyl or alkenyl groups.
- the sugar unit is selected from the group of aldoses and ketoses, i.e. furanoses and pyranoses, preferably glucoses.
- alkylpolyglycosides In principle, it is possible to convert known polysaccharides, oligosaccharides and monosaccharides such as starch, maltodextrin, dextrose, galactose, mannose and xylose into alkylpolyglycosides.
- Industrially accessible carbohydrates such as starch, maltodextrin and dextrose are preferred as precursors. Since the industrially relevant alkylpolyglycoside syntheses are not directed to the selection of regioselectively or stereoselectively preferred derivatives, the alkylpolyglycolsides are in majority mixtures of monomers and oligomers which in turn are mixtures of different isomeric structures.
- alkylpolyglycosides can also contain residues of associated substances such as alcohols, monosaccharides, oligosaccharides and oligoalkylpolyglycosides.
- the formula (II) therefore represents all types of carbohydrates, monomers and polymers including furanoses and pyranoses, such as
- A (for the alkylpolyglycoside formulas) is selected from the group of straight- or branched-chain, C 4 -C 2 4-alkyl or alkenyl groups preferably C 8 -C 2 4-alkyl or alkenyl groups or a mixture of straight- or branched-chain, C 4 -C 24 -alkyl or alkenyl groups preferably C 8 -C 24 -alkyl or alkenyl groups;
- Z is selected from the group of hydrogen, C 1 -C 4 -alkyl or alkenyl groups or a mixture of straight- or branched-chain, C C 4 -alkyl or alkenyl groups;
- R 31 is selected from the group of hydrogen, C - C 10 -alkyl or alkenyl groups or a mixture of straight- or branched-chain, Ci - C 8 -alkyl or alkenyl groups.
- the alkyl or alkenyl radical A may be preferably derived from compounds such as primary alcohols containing 4 to 22 and preferably 8 to 18 carbon atoms. Typical examples are butanol, pentanol, caproic alcohol, caprylic alcohol, capric alcohol and undecyl alcohol and the technical mixtures thereof obtained, for example, in the hydrogenation of technical fatty acid, fatty acid derivatives, e.g. methyl esters, Ziegler alcohols or in the hydrogenation of aldehydes from ' Roelen's Oxo synthesis ' .
- Further alcohols include lauryl alcohol, myristyl alcohol, cetyl alcohol, palmitoleyl alcohol, stearyl alcohol, isostearyl alcohol, oleyl alcohol, elaidyl alcohol, petroselinyl alcohol, arachyl alcohol, gadoleyl alcohol, behenyl alcohol, erucyl alcohol, brassidyl alcohol and technical mixtures thereof which may be obtained as described above.
- the index ' p ' in the general formula (II) indicates the degree of polymerization (P n ) calculated on the basis of average number of molecular weight, i.e. represents the distribution of mono- and oligoglycosides.
- the index ' p ' is a number from 1 to 15.
- the degree of polymerization P n or index p ' in an individual compound is an integer and, above all, may have a preferred value of 1 to 6, the value ' p ' for a commercial alkyl oligoglycoside is an analytically determined calculated value which as a result is generally a fractional number.
- Alkyl and/or alkenyl oligoglycosides having an average degree of oligomerization ' p ' of 1.1 to 3.0 are preferably used.
- the alkylpolyglycosides used in this invention can be prepared partly or entirely on the basis of renewable raw materials by known synthesis. For example, poly- or oligosaccarides are reacted in the presence of an acidic catalyst with n- pentanol to form pentyl oligoglycoside mixtures which are then converted to the desired alkyloligoglycosides with long-chain alcohols in the presence of an acidic catalyst. Usually a composition of several compounds having a distribution regarding the glycosidic and the alkyl component is obtained and used respectively.
- alkylpolygylcosides Another synthetic approach to the alkylpolygylcosides is to obtain fatty alcohols or mixtures thereof from natural fatty esters, like coconut oil, by transesterification, hydrogenation and subsequent distillation, and to react them with glucose or other hydrolysis products from starch.
- alkylpolygylcosides of formula (II) defined above, used in this invention, are preferably represented by the products sold by the Companies Henkel, Sasol and Cognis under the name APG, such as the products APG 300, APG 350, APG 500, APG 550, APG 625, APG base 10-12, Plantacare® or Glucopon®, the products sold by the company BASF under the name LUTENSOL® GD 70 e.g., sold by Seppic under the names TRITON® CG 110 (or ORAMIX ⁇ CG 1 10) and TRITON® CG 312 (or ORAMIXNS 10).
- APG the products sold by the Companies Henkel, Sasol and Cognis under the name APG, such as the products APG 300, APG 350, APG 500, APG 550, APG 625, APG base 10-12, Plantacare® or Glucopon®
- LUTENSOL® GD 70 e.g., sold by Seppic under the
- the preferred alkylpolyglycosides of formula (II), according to the invention, are APG 300 of the Company Henkel, TRITON® CG 1 10 of the Company Seppic and LUTENSOL® GD 70 of the Company BASF, especially preferred Plantacare® 2000 and Glucopon® 600 or 215.
- Alkyloligoglucosides based on hydrogenated C12/C14-coconut alcohol with a P n of 1 to 3 are preferred.
- Alkylpolyglycosides are described for example in DE-A-1 943 689, EP-A-418479 or EP-A-1972817.
- the aqueous emulsions according to the present invention comprise one or more ionic co-surfactants c).
- Ionic surfactants include surfactants that have ionic or charged groups, like ammonium, phosphonium, sulfate, carboxylate, sulfonate, phosphonate, and suitable counter ion or counter ionic groups, including metal or non-metal cations, acid anions, like halogen anions, acetate, tosylate, sulfate, phosphate, nitrate etc.
- Component c) include one or more co-surfactants selected from the group consisting of amphoteric or zwitterionic surfactants, amine oxide surfactants and cationic surfactants.
- co-surfactants c) are selected from amphoteric or zwitterionic and cationic surfactants, having preferably more than 6 carbon atoms.
- Cationic surfactants include for example nonpolymerized, organic, quaternary ammonium compounds, preferably hydrocarbon-containing quaternary ammonium salts or amine salts, where the hydrocarbon groups may preferably contain from 8 to 28 carbon atoms.
- the R 12 , R 13 , R 14 and R 15 groups may each independently contain one or more, preferably two, ester (-[-O-C(O)-]; [-C(0)-0-]) and/or amido groups ( ⁇ [CO-N(R 12 )-]; [-N(R 12 )-CO-]) in which R 12 is as defined above.
- the anion X may be selected from halides, methosulfate, acetate and phosphate, preferably from halides and methosulfate.
- component c) are tetraorgano-substituted quaternary ammonium compounds having one or two long-chain C8 to C28 hydrocarbon radicals and two or three short-chain C1 to C6 hydrocarbon radicals.
- the long- chain radicals are preferably C12 to C20 chains and the short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and hydroxyethyl, 2-hydroxypropyl.
- Preferred counterions are CI " , Br " , CH 3 OS0 3 ⁇ , C 2 H 5 OS0 3 ⁇ N0 3 " , HCOCr and CH 3 COO ⁇
- Examples include: dodecylethyldimethylammonium bromide
- the chain length of the long-chain hydrocarbon group is preferably from 12 to 15 carbon atoms, and the short-chain radicals R 13 , R 14 and R 15 are preferably methyl and hydroxyethyl.
- the chain length of the long-chain hydrocarbon groups is preferably from 12 to 28 carbon atoms.
- Preferred ester-containing surfactants have the formula:
- R 16 is independently selected from Ci -4 alkyl, hydroxyalkyl or C 2-4 alkenyl; and in which R 17 is independently selected from Cs-28 alkyl or alkenyl groups;
- E is an ester group, i.e. -OC(O)- or -C(0)0-, z is an integer from 0 to 8 and X " is as defined above.
- They contain two or three short-chain C1 to C6 hydrocarbon radicals.
- the one or two long alkyl radicals per molecule derive from fatty acids of lengths from C8 to C26, preferably from C10 to C20, especially from C12 to C18.
- the fatty acids or the cuts of the chain length ranges mentioned may be saturated fatty acids, unsaturated fatty acids, hydroxy-substituted fatty acids or mixtures thereof.
- Examples of the acids mentioned are lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and ricinoleic acid. Further examples are tallow fatty acid and coconut fatty acid cuts. They are bonded to the quater- nized nitrogen preferably via oxyethyl, 2-oxypropyl or 1 ,2-dioxypropyl or oligooxyethylene spacers.
- the short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and hydroxyethyl, 2-hydroxypropyl.
- Preferred counterions are CI “ , Br “ , CH 3 OS0 3 “ , C 2 H 5 OS0 3 “ , N0 3 “ , HCOO " and CH 3 COO " . Examples include
- a further type of preferred ester-containing cationic surfactants can be represented by the following formula:
- a further group of cationic surfactants c) is those of the formula R 18 A(CH 2 ) 2 - 4 NR 19 R 20 in which R 18 is C 6 -C 12 alkyl;
- A is a divalent group which is selected from -NH-, -CONH-, -COO- or -0-, or A may be absent.
- R 19 and R 20 are each independently selected from the group consisting of H, C Ci 4 alkyl or (CH 2 -CH 2 -0(R 21 )) in which R 21 is H or methyl.
- Particularly preferred surfactants of this type are decylamine, dodecylamine, C 8 -C 12 bis(hydroxyethyl)amine, C 8 -Ci 2 bis(hydroxypropyl)amine, C 8 -C 2 amidopropyldimethylamine or salts thereof.
- the one or two long alkyl radicals per molecule derive from fatty acids of lengths C 8 to C 2 6, preferably C 10 to C 2 o, especially C 12 to Ci 8 .
- the fatty acids or the cuts of the chain length ranges mentioned may likewise be saturated fatty acids, unsaturated fatty acids, hydroxy-substituted fatty acids or mixtures thereof.
- Examples of the acids mentioned are lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and ricinoleic acid. Further examples are tallow fatty acid and coconut fatty acid cuts. They are bonded to the quaternized nitrogen preferably via amidoethyl and 3-amidopropyl spacers.
- the short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and hydroxyethyl, 2-hydroxypropyl. They may also alternatively be cyclic radicals such as imidazolinium radicals into which fatty alkyl substituents have optionally additionally been incorporated.
- Preferred counterions are CI “ , Br “ , CH3OSO3 , C2H5OSO3 " , N0 3 " , HCOO " and CH 3 COO " .
- amine salts may also find use. These are salts of primary, secondary or tertiary amines with inorganic or organic acids.
- the nitrogen is substituted by one or two long- chain C 8 to C 2 8 hydrocarbon radicals, one to three hydrogen atoms and optionally one or two short-chain Ci to C6 hydrocarbon radicals.
- the one or two long alkyl radicals per molecule derive, for example, from fatty amines or fatty acids of lengths C 8 to C 2 e, preferably Ci 0 to C 20 , especially C12 to Cis atoms.
- Preferred counterions are CI “ , Br “ , CH3OSO3 " , C 2 H 5 OS0 3 " , N0 3 " , HCOO " and CH 3 COO " .
- the fatty acids or the cuts of the chain length ranges mentioned may be the saturated fatty acids, unsaturated fatty acids, hydroxy-substituted fatty acids or mixtures thereof, which have already been described.
- Examples of the acids mentioned are lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and ricinoleic acid. Further examples are tallow fatty acid and coconut fatty acid cuts. They are bonded to the amine salt nitrogen preferably via oxyethyl,
- the short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and hydroxyethyl, 2-hydroxypropyl.
- Preferred counterions are CI “ , Br “ , CH3OSO3 " , C 2 H 5 OS0 3 " , N0 3 " , HCOO " and CH3COO " .
- Suitable amphoteric or zwitterionic surfactants are described for example in US 5, 104,646 , 5, 106,609 . They include those surfactants broadly described as derivatives of aliphatic secondary and tertiary amines in which the aliphatic radical can be straight or branched chain and wherein one of the aliphatic substituents contains from 8 to 18 carbon atoms and one contains an anionic group such as carboxy, sulfonate, sulfate, phosphate, or phosphonate.
- Suitable amphoteric surfactants for use in the present invention include cocoampho- acetate, cocoamphodiacetate, lauroamphoacetate, lauroamphodiacetate, and mixtures thereof.
- Further zwitterionic surfactants suitable for use in the compositions are well known in the art, and include those surfactants broadly described as derivatives of aliphatic quaternary ammonium, phosphonium, and sulfonium compounds, in which the aliphatic radicals can be straight or branched chain, and wherein one of the aliphatic substituents contains from 8 to 18 carbon atoms and one contains an anionic group such as carboxy, sulfonate, sulfate, phosphate or phosphonate.
- Further zwitterionic surfactants include betaines, and furthermore, although less preferred: amine oxide surfactants like Ci 2 -Ci 4 .alkyldimethyl amine oxide.
- Non-limiting examples of other ionic additional surfactants suitable for use in the compositions are described in McCutcheon's, Emulsifiers and Detergents, 1989 Annual, published by M. C. Publishing Co., and US 3,929,678, US 2,658,072; US 2,438,091 ; US 2,528,378.
- the co-surfactants used in the aqueous emulsions according to the invention are preferably essentially free of (poly)oxyalkylene groups.
- the entire aqueous emulsions according to the invention are essen tially free of (poly)oxyalkylene compounds, that is, compounds that have two or more oxyalkylene groups.
- Essentially free means that the aqueous emulsions contain less than 1 % by weight more preferably less than 0.5 % by weight based on the entire emulsion.
- the weight ratio of the ionic co-surfactants to the alkyl polyglucosides is between 0.001 and 0.5.
- the aqueous emulsions according to invention comprise 0.1 - 50 parts of one or more ionic co-surfactants, based on 100 pw of the polyorganosiloxanes.
- the preferred co-surfactants of the instant invention may include ionic organic surfactants that contain at least one Ci 0 - C 30 -alkyl or alkylaryl group and at least one ionic hydrophilic group.
- ionic organic surfactants that contain at least one Ci 0 - C 30 -alkyl or alkylaryl group and at least one ionic hydrophilic group.
- cosurfactants that have cationic, amphoteric, zwitterionic or (less preferred) amine oxide structures.
- Most preferred are cosurfactants that do not contain any oxyalkylene groups.
- cationic surfactants include quaternary ammonium compounds and their salts, such as tallowtrimethylammonium chloride, dodecyltrimethyl ammonium chloride, hexadecyltrimethylammonium chloride, didodecyldimethylammonium chloride, dicocoalkyldimethylammonium chloride, n-alkyldimethylbenzeneammonium chlorides, as well as the corresponding hydroxides, acetates or fatty acid salts.
- Other examples are the primary, secondary or tertiary aliphatic amine salts such as dodecylammonium acetate, alkyldimethylammonium chloride or the like.
- alkyltrimethylammnium chlorides or dialkyldimethylammonium chlorides such as hexadecyltrimethylammonium chlordie and dicocoalkyldimethylammonium chloride, or the corresponding hydroxides or acetates.
- Preferred examples of zwitterionic surfactants are amino acid based surfactants and related betaine surfactants, preferred examples of which are cocoalkylamido- propylbetaine, N-lauryldimethylbetaine, cocoalkylamidopropylsulfobetaine, 3- (dodecyldimethylamonio)-propansulfonate and the like.
- cocamidopropyl betaine of the formula:
- Component c) includes also amine oxides.
- Suitable amine oxide surfactants have tho fnrmi ⁇
- R is identical or different, and is a straight or branched optionally substituted alkyl group, wherein one or more methylene groups might be replaced by O, N H , and optional substituents may include 1 to 3 substituents, including hydroxyl etc.
- Non-limiting examples of suitable amine oxide compounds include dimethyl- dodecylamine oxide, oleyldi(2-hydroxyethyl) amine oxide, dimethyltetradecyl- amine oxide, di(2-hydroxyethyl)-tetradecylamine oxide, dimethylhexadecylamine oxide, behenamine oxide, cocamine oxide, decyltetradecylamine oxide, dihydroxyethyl Ci 2 -Ci 5 -alkoxypropylamine oxide, dihydroxyethyl cocamine oxide, dihydroxyethyl lauramine oxide, dihydroxyethyl stearamine oxide, dihydroxyethyl tallowamine oxide, hydrogenated palm kernel amine oxide, hydrogenated tallowamine oxide, hydroxyethyl hydroxypropyl Ci 2 -Ci 5 -alkoxypropylamine oxide, lauramine oxide, myristamine oxide, myristyl/cetyl amine oxide, oleamidopropy
- amine oxides are alkylamine oxides or alkyldimethylamine oxides, such as lauryldimethylamine oxide, stearyldimethyl- amine oxide or cocosalkyldimethylamine oxide and the like.
- the aqueous emulsions according to invention comprise 100 - 1000 parts by weight preferably 150 to 800 parts by weight, more preferably 200 to 600 parts by weight of water based on 100 parts by weight of the component a), i.e. the polyorganosiloxanes.
- aqueous emulsions Due to the specific composition of the aqueous emulsions according to the invention, they are usually optically clear.
- the components a) to c) are finely divided as droplets in the aqueous phase.
- the droplets have a volume average d 50 size of less than 120 nm, preferably less than
- the aqueous emulsions according to the invention may contain water soluble compounds such as glycerine, low molecular weight alcohols, organic or inorganic salts, amines and the like. Additionally, these emulsions may contain additives such as biocides, perfumes, dies, pigments, thickeners or fillers. Preferred are emulsions that contain less than 5 % by weight such additives. Most preferred are emulsions should contain less than 3 % by weight additives and less than 1 % by weight total biocides. Additionally, the pH of the emulsions may be controlled using common acids, bases, like Methanol amine, common amino acids and the corresponding buffers.
- microemulsions according to the invention form spontaneously upon mixing without the use of high shear and are characterized by an average particle size of preferably less than 120 nanometers causing the emulsions to be optically clear.
- inventive microemulsions are stable to dilution with water to less than 2 parts polyorganosiloxane in 100 parts of water, without phase separation.
- inventive aqueous emulsions may also comprise other oil soluble compounds such as paraffin oil, non-polar cyclic siloxanes or polyorganosiloxanes, or organic alcohols, amines, esters or ketones.
- oil soluble compounds such as paraffin oil, non-polar cyclic siloxanes or polyorganosiloxanes, or organic alcohols, amines, esters or ketones.
- emulsions that comprise oil phases containing at least 20 % by weight of one or more polyorganosiloxanes a) containing polar pendant groups.
- aqueous emulsions according to the invention can be used in particular for the manufacture of cosmetic compositions, in particular also for transparent cosmetic compositions, for example for the manufacture of hair care compositions, such hair shampoos, hair gels, hair conditioners, skin care compositions such skin cleansing compositions, skin cleansing gels, carrier such as ointment basis for bioactive ingredients such as cosmetically or pharmaceutically active ingredients, carrier for chemicals in general such as active ingredients, such as pharmaceuticals, agrochemicals such as herbicides, insecticides, fungicides etc.
- they can be used for treatment of fibers or fibrous materials, such as initial textile treatment, in particular to provide antistatic and/or softening properties to textile fibers, paper or paper tissue, non- wovens, leather or furs.
- inventive emulsions can be used in the treatment of carbon or glass fibers or substrates. Furthermore, the inventive emulsions can be used in the treatment of painted surfaces, glass or plastic surfaces, for examples car bodies or windshields, rendering these surfaces more hydrophobic and/or more resistant to weathering and cleansing or polishing agents. Furthermore, the inventive emulsions can be used alone or formulated with other materials in the treatment of materials used in construction and civil engineering where water repellency is desired. This includes materials such as masonry and wood products, such as brick, paving materials, asphalt, cement, plaster, molding, roofing tiles, stucco, porcelain, stone, marble, tiles, adobe, concrete, masonite, mineral board, particle board, gypsum and so forth.
- the treated construction materials are characterized by improved water repellency or water-proofness and/or improved resistance to weathering.
- the aqueous emulsions can be used for surface treatment, for example in cleansing, polishing or disinfection compositions. In all these applications it is an advantage of the aqueous emulsions that they are optically clear or transparent.
- the aqueous emulsions according to the invention are preferably optically clear ' oil-in-water ' emulsions
- the mixture was acidified to a pH value of 5 with 1.2 g of acetic acid and heated to 70 °C for 30 minutes, resulting in a clear microemulsion.
- Neutralisation with 2,0 g of a 60 % triethanolamine solution gave a final pH value of 8.
- 1 ,0 g of a 30 % actives solution of parabenes in 2-phenoxyethanol was added as a preservative.
- the resulting product was a optically clear emulsion with an average particle size of 65 nm, a silicone content of 20 wt.-%, a total solids content of
- the mixture was acidified to a pH value of 5 with 1 .3 g of acetic acid, resulting in a clear microemulsion.
- Neutralization with 2.0 g of a 60 % triethanolamine solution gave a final pH value of 8.
- 1 ,0 g of a 30 % actives solution of parabenes in 2- phenoxyethanol was added as a preservative.
- the resulting product was a optically clear emulsion with an average particle size of 66 nm, a silicone content of 20 %, a total solids content of 38.9 %.
- the emulsion was also stable to dilution with a 10 % solution of sodium carbonate.
- the resulting product was optically clear, with a light yellow color, an average particle size of 87 nm, a silicone content of 15 %, a total solids content of 33.85 % and a viscosity at 23 °C of 8.15 mPa * s.
- the emulsion was unchanged. Diluting the emulsion 5:2 with 10% sodium chloride solution gave no change.
- the emulsion was also stable to neutralization with either triethanolamine or sodium carbonate to a pH value of 8. In each case the resulting neutralized emulsion was clear and stable at 40 °C for in excess of 6 weeks.
- Diluting the emulsion 1 :9 with deionized water gave a clear emulsion that remained stable both at room temperature and at 40 °C for in excess of 4 weeks.
- a clear shampoo was formulated with 15.4 % of Plantacare 2000 UP, a 50 % solution of alkylpolyglucosides, 7.8 % of a 33 % actives solution of cocoamido- propyl betaine, 5.2 % of a sodium Ci 2 -Ci 8 -alkylsulfate (available as Sulfopon 1216G from the Cognis), 1 .5 % Xanthan Gum, 5.0 % of the inventive emulsion from Example 2, 0.5 % citric acid and water.
- the resultant shampoo was applied to standardized hair switches in comparison with the same formulation, but without silicone. Hair treated with the inventive shampoo was soft to the touch and very easy to comb. The hair treated with the silicone-free shampoo was noticeably less soft to the touch and could only be combed with difficulty.
- a clear hair conditioner was formulated with 1.5 % cetearyl alcohol, 0.3 % cetrimonium chloride, 1.5 % hydroxyethylcellulose, 1 .0 % glyceryl stearate, 5.0 % of the inventive emulsion from Example 2 and water.
- the resultant hair conditioner was a clear, viscous liquid.
- the formulation was applied to standardized hair switches that had been washed with a conditioner-free standard shampoo. Hair treated with the inventive conditioner was soft to the touch and very easy to comb. The untreated, washed hair was noticeable less soft to the touch and could only be combed with difficulty.
- Emulsion of Siloxane (2), alkylpolyglycoside and 1-pentanol with an APG/Siloxane ratio of 0.35 (according to US 5,466,746, Example 6)
- the resulting emulsion Upon standing at room temperture the resulting emulsion had an average particle size of 204 nm, was opaque, had a silicone content of 15 %, and a total solids content of 32.84 %. Diluting the emulsion 1 :9 with deionized water gave an opaque macroemulsion.
- the resulting microemulsion was completely clear, but slightly yellow. Neutralization with sodium carbonate to a pH value of 8 caused phase separation. Diluting the emulsion 1 :9 with deionized water gave a clear emulsion that remained stable both at room temperature and at 40 °C for in excess of 4 weeks. Diluting the emulsion 5:2 with 10% sodium chloride solution gave no change.
Abstract
The invention relates to optically clear acqueous emulsions, comprising certain polyorganosiloxanes, alkylpolyglucosides, specific co-surfactants and water, and the use thereof.
Description
AQUEOUS EMULSIONS OF POLYORGANOSILOXANES
This instant invention pertains to clear aqueous microemulsions of polyorganosiloxanes containing polar groups and alkylpolyglycosides. The emulsions of this invention are free of polyglycols and surfactants with oxyalkylene functional groups.
BACKGROUND
Aqueous emulsions of polyorganosiloxanes are well known. Clear microemulsions of polysiloxanes are less common and their preparation is limited to specific polyorganosiloxanes with polar pendant groups and specific limiting combinations of surfactants and specific process steps, see US 4, 146,499, US 4,620,878, US 5,057,572, US 5,244,598, US 5,258,451 , US 5,302,657, US 5,683,625, US 6, 147,038, US 6,451 ,905, and US 6,547,490. The microemulsion compositions described all use organic ethoxylates as surfactants and do not describe the use of alkylpolyglycosides (APGs).
Aqueous emulsions of polyorganosiloxanes are used in many applications, for example, as release agents, slip agents, water repellants, in the treatment of textiles and other fibrous materials and in detergents, cosmetic and other derma tological formulations for personal care. The use of emulsions of polyorganosiloxanes containing polar pendant groups in personal care formulations is well documented, for example, in US 4,529,586, US 4,563,347, US 4,559,227, US 4,563,347 and US 4,586518.
Personal Care formulations are also known (US 5,035,832, US 5,409,628, DE 4,229,922 and US 5,690,920) which claim the use of both silicones and alkylpolyglycosides, but in none of these cases do the alkylpolyglycosides serve the purpose of emulsifying the polysiloxane and the polysiloxane is not claimed to be a microemulsion.
The use of clear aqueous microemulsions of polyorganosiloxanes in personal care formulations have been described in US 6, 153,569 and US 6, 147,038, but these documents do not describe the use of alkylpolyglycosides to emulsify the polysiloxane.
The US 5,268, 126 and EP 418479 describe the use of alkylpolyglycosides as surfactants in the preparation of emulsions of polysiloxanes, but do not describe the preparation of clear microemulsions.
The US 5,466,746 describes the use of alkylpolyglycosides to make emulsions with the composition: a) 100 parts by weight of polyorganosiloxanes which contain polar groups on Si-C-bonded hydrocarbon radicals, with the proviso that methyl or phenyl groups are not to be included with the Si-C-bonded hydrocarbon radicals which contain polar groups, and
b) not more than 50 parts by weight of alkylpolyglycosides, and
c) 0-30 % of a cosurfactant, and
d) water.
Whereby the claimed cosurfactants are C4-C8-alcohols, glycol ethers, esters or ketones. These compositions do not spontaneously give rise to microemulsions, but as a rule require the use of high shear forces. Microemulsions are also only obtained in the presence of one of the claimed cosurfactants.
The DE 41 31 551 describes the use of alkylpolyglycosides to obtain aqueous dispersions of amino- or amido substituted polyorganosiloxanes for the treatment of fabrics. The compositions comprise:
a) 10-25 parts polyorganosiloxanes, which contain amino or amido groups on Si-C bonded hydrocarbon radicals.
b) 3-15 parts surfactant and
c) 60-87 parts water
whereby at least one of the surfactants in b) is an alkylpolyglycoside. Possible cosurfactants are defined as fatty alcohol ethoxylates and the like. Preferred dispersions contain only alkylpolyglycoside surfactants. Microemulsions are obtained by acidification of the emulsion according to WO 98/008436. The stability of these dispersions towards neutralization and/or electrolytes is not described. It has been observed that the preferred microemulsions comprising only alkylpolyglycosides do not exhibit the desired stability towards neutralization or electrolytes.
In particular, it was found that microemulsions of polyorganosiloxanes containing polar pendant groups could not be obtained according to the method of DE 4131551 with alkylpolyglycosides as the only surfactants, if the content of polar pendant groups is low. High contents of polar pendant groups are commonly to be avoided as these groups can easily be oxidized resulting in coloration (yellowing) in the end use product. This is particularly true of aminosubstituted polyorganosiloxanes having pendant amino groups of type a2). It is, therefore, desirable to be able to make polyorganosiloxanes a2) with less than 0.9 mmol/g polar pendant groups, whereas this concentration relates to polydimethylsiloxanes and has to be adjusted accordingly the molweight of other polyorganosiloxanes where methyl groups are replaced by other organo groups. It is especially desirable to be able to make microemulsions of polyorganosiloxanes with between 0.3 and 0.9 mmol/g polar pendant groups.
Microemulsions of silicones a2) with polar pendant groups are particularly desirable raw materials in the preparation of personal care formulations for hair and skin care. These microemulsions are commonly formulated with additional surfactants, other oil phases and water. The resulting formulation is as a rule buffered to a neutral pH and may contain added electrolytes as thickening
agents. A key requirement of these microemulsions is that they have to be stable to dilution with water, pH shifts and/or added electrolytes.
Currently these requirements are achieved by use of surfactants that contain organic ethoxylates with oxyalkylene functional groups, such as described in US 4,620,878, US 4, 146,499 or US 4,620,878. These surfactants are known to be eye and skin irritants and it is desirable to avoid their use in personal care formulations. There is, therefore, the need for stable microemulsion compositions, which are stable against dilution, change of pH and addition of electrolyte comprising polyorganosiloxanes with polar pendant groups that are prepared without the use of organic ethoxylates.
OBJECTIVE:
The objective of the instant invention is to provide optically clear microemulsions of polyorganosiloxanes containing polar groups, for example, pendant polar groups, which can be made with a minimum of shear energy and which are stable to dilution with water, neutralization and addition of electrolytes. In another aspect, the instant invention provides clear microemulsions that are free of polyglycols, organic ethoxylates and other surfactants with oxyalkylene functional groups.
Surprisingly, it was found that combining certain polyorganosiloxanes with one or more alkylpolyglycosides and, in particular, specific organic co surfactants leads to the spontaneous formation of stable, clear microemulsions. The use of non- ionic alcohol ethoxylates according to DE 41 31 551 leads to stable microemulsions, but replacement of these cosurfactants with other non-ionic surfactants that do not contain alkylene oxide groups did not yield stable microemulsions. It was found that in particular polar ionic organic co-surfactants taken from the group consisting of amphoteric or zwitterionic surfactants, alkyl amine oxides and cationic surfactants gave stable microemulsions. Other ionic
and non-ionic cosurfactants that do not contain alkylene oxide groups do not give stable microemulsions of polyorganosiloxanes containing polar pendant groups. The list of cosurfactants that do not give stable microemulsions includes but is not limited to sorbitan and glycerin esters of fatty acids, and anionic surfactants such as alkyl sarcosinates and their salts, alkyl isethionates and their salts, as well as alkyl sulfonates and their salts. Without being bound by theory it is believed that the positive charge or dipole in the surfactant structure is required to obtain stable microemulsions of polyorganosiloxanes, in particular, those containing polar pendant groups.
This is the more surprising as the inventive microemulsions form spontaneously by simple mixing and the properties of the emulsions are independent of the order of addition, the pH of the emulsion or the shear force used in their preparation. These microemulsions are extraordinarily stable, remaining unchanged by storage at elevated temperatures or shifts in pH from acid to neutral or basic. The microemulsions are also stable to dilution with water and addition of electrolytes such as sodium chloride.
DETAILED DESCRIPTION
The instant invention describes aqueous, finely divided, optically clear oil-in-water silicone emulsions comprising: a) 100 parts by weight of one or more polyorganosiloxanes which contain one or more polar groups on Si-C-bonded hydrocarbon radicals, b) > 50 - 150 parts by weight of one or more alkylpolyglucosides, c) 0.1 - 150 parts by weight of one or more co-surfactants selected from the group consisting of amphoteric or zwitterionic surfactants, amine oxide surfactants and cationic surfactants, d) water,
preferably a) 100 parts by weight of one or more polyorganosiloxanes which contain one or more polar groups on Si-C-bonded hydrocarbon radicals, b) > 50 - 150 parts by weight of one or more alkylpolyglucosides, c) 0.1 - 150 parts by weight of one or more co-surfactants selected from the group consisting of amphoteric or zwitterionic surfactants, amine oxide surfactants and cationic surfactants, d) 100 - 1000 parts by weight water, more preferably a) 100 parts by weight of one or more polyorganosiloxanes which contain one or more polar groups on Si-C-bonded hydrocarbon radicals, b) > 50 - 150 parts by weight of one or more alkylpolyglucosides,
c) 0.1 - 60 parts of one or more co-surfactants, 0.1 - 150 parts by weight of one or more co-surfactants selected from the group consisting of amphoteric or zwitterionic surfactants, and cationic surfactants,
d) 100 - 1000 parts by weight water.
Component a) is preferably a polyorganosiloxane, in which the polar groups bonded to Si-C-bonded hydrocarbon radicals are selected from the group consisting of amino, ammonium, epoxy, hydroxyl, amido, mercapto, carboxyl, carboxylic acid ester, sulphonic acid, sulphonic acid ester groups or salts thereof, preferably from amino and ammonium groups.
Preferred polar groups are amino groups and/or ammonium groups, including:
Monofunctional polar groups: -NH2, -NH3 +, -NHR, -NH2R+, -NHR2 +, -NR2, -NR3 +, wherein R is an organic group, in particular, an optionally substituted C -C22-alkyl group, wherein one or more methylene groups are replaced by -NH-, -0-, -CO-, -
NR-. Substitutents of the Ci-C22-alkyl group include in particular 1 to 3 groups selected from hydroxy and -NH2, These monofunctional amino or ammonium groups are preferably used in polyorganosiloxanes a2) that have pendant polar groups, attached to silicone atoms of a polydiorganosiloxane moiety via a hydrocarbon residue. Such polyorganosiloxanes a2) that have pendant polar groups are described more specifically below.
Di- or polyfunctional polar groups, including groups like -NH-, - H2 +-, -NR-, - NHR+-, -NR2 +-, wherein R is an organic group, are in particular used in the polyamino- and/or polyammonium-polysiloxane compounds a1 ) as described in the following. In such polysiloxane compounds, the polar group is in general part of the molecule backbone formed of polydiorganosiloxane moieties and polar group-containing moieties, which are attached to the polydiorganosiloxane moieties via hydrocarbon residues, comprising a SiC-bond.
Component a) includes, in particular, polyamino- and/or polyammonium-polysiloxane compounds a1 ) and/or polar polysiloxane compounds a2) having pendant polar groups preferably amino- and/or ammonium groups.
The polyamino- and/or polyammonium-polysiloxane compound a1 ) is a copolymer compound, which has amino and/or ammonium repeat units and polysiloxane repeat units in the polymer main chain. The amino units contain secondary and/or tertiary nitrogen atoms (2 or 3 organic radicals on the uncharged nitrogen atom). The ammonium units contain secondary, tertiary and/or quaternary, positively charged nitrogen atoms (2, 3 or 4 organic radicals on the nitrogen). The amino and/or ammonium repeat units may also serve heterocyclic radicals bonded into the polymer chain via two nitrogen atoms.
In contrast, polysiloxane compounds a2) have pending polar groups as defined above. Preferably these polar groups are pendant amino- and/or ammonium groups in polysiloxane compounds showing the structure a2) below. The compounds a2) contain polar groups preferably amino and/or ammonium groups in the pendant groups of the polysiloxane main chain. The pendant groups are
linked over Si-C bonds to the Si atoms. In other words, the amino and/or ammonium groups are not present in the main chain comprising the polysiloxane repeat units.
amino- and/or ammonium-polysiloxane compound a2):
— Polyorganosiloxan chain—
Amino/Ammonium-containing radical
In the inventive formulation, components a1 ) and a2) may be present alone or together. In a preferred embodiment, component a2) is, however, present alone in the inventive formulation, without component a1 ). In another preferred embodiment, both component a1 ) and component a2) are present together.
Components a1 ) or a2) may be present together in any ratios relative to one another.
The polyamino- and/or polyammonium-polysiloxane compound a1 ) preferably comprises polysiloxane compounds which contain at least one unit of the formula (I):
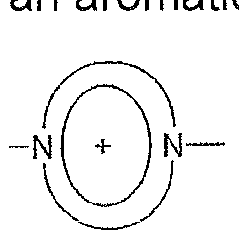
-[Q-V]- (I) in which Q is selected from the group consisting of:
-NR-,
-N + N— a trivalent radical of the formula:
/
—
\
a trivalent radical of the formula:
/
— N+
■ or
in which R is in each case hydrogen or a monovalent organic radical, where Q is not bonded to a carbonyl carbon atom,
V is at least one constituent which is selected from the group consisting of V1 , V2 and V3, where
V2 is selected from divalent, straight chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radicals which have up to 1000 carbon atoms (not counting the carbon atoms of the polysiloxane radical Z2 defined below) and may optionally contain one or more groups selected from
-0-, -CONH-,
-CONR2-, in which R2 is hydrogen, a monovalent, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 100 carbon atoms, may contain one or more groups selected from -0-, -NH-, -C(O)- and -C(S)-, and may optionally be substituted by one or more substituents selected from the group consisting of a hydroxy I group, an optionally substituted heterocyclic group preferably containing one or more nitrogen atoms, amino, alkylamino, dialkylamino, ammonium, polyether radicals and polyether ester radicals, where, when a plurality of - CONR2 groups is present, they may be the same or different,
-C(O)- and -C(S)-, the V2 radical may optionally be substituted by one or more hydroxy I groups, and the V2 radical contains at least one -Z2- group of the formula
in which
R1 may be the same or different and is selected from the group consisting of: Ci to C22-alkyl, fluoro(d-C10)-alkyl and C6-C10-aryl, and n-i = from 20 to 1000,
V1 is selected from divalent, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radicals which have up to 1000 carbon atoms and may optionally contain one or more groups selected from
-0-, -CONH-,
-CONR2-, in which R2 is as defined above, where the R2 groups in the V1 and V2 groups may be the same or different,
-C(O)-, -C(S)- and -Z1-, where -Z - is a group of the formula
in which
R1 is as defined above, where the R1 groups in the V1 and V2 groups may be the same or different, and
n2 = from 0 to 19, and the V1 radical may optionally be substituted by one or more hydroxyl groups, and
V3 is a trivalent or higher-valency, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 000 carbon atoms, may optionally contain one or more groups selected
from
-0-, -CONH-, -CONR2-, in which R2 is as defined above, -C(O)-, -C(S)-, -Z1- which is as defined above, -Z2- which is as defined above and Z3, where Z3 is a trivalent or higher-valency polyorganosiloxane unit, and may optionally be substituted by one or more hydroxy I groups, where, in said polysiloxane compound, in each case one or more V1 groups, one or more V2 groups and/or one or more V3 groups may be present, with the proviso
- that said polysiloxane compound contains at least one V1 , V2 or V3 group which contains at least one -Z1-, -Z2- or Z3 group, and
- that the tri- and tetravalent Q radicals either serve to branch the main chain formed from Q and V, so that the valencies which do not serve for bonding in the main chain bear further branches formed from -[Q-V]-units, or the tri- and tetravalent Q radicals are saturated with V3 radicals within a linear main chain without formation of a branch, and wherein the positive charges resulting from ammonium groups are neutralized by organic or inorganic acid anions, and acid addition salts thereof.
The polysiloxane compounds which contain at least one unit of the formula (I) are terminated by monofunctional -Q-R and/or -V-R groups, i.e., for example, by amino groups. These arise by saturation of one of the two binding points of Q or V by a monovalent R group or hydrogen, which is as defined above, and are also referred to below as Vst or Qst. Instead of Vst, other unconverted reactive groups such as epoxy or haloalkyl groups may also be present.
In the context of the invention, the polysiloxane compounds which contain at least one unit of the formula (I) are also intended to include the case where only one -[Q-V]- unit is present, so that compounds of the formula
R-V-[Q-V]-R or R-[Q-V]-Q-R, where R may also be replaced by H, are also included.
Suitable polyamino- and/or polyammonium-polysiloxane compounds a1 ) are described, for example, in WO 02/10257, WO 02/10259, DE-A 100 36 522, DE-A 100 36 532, DE-A 100 36 533 and the unpublished DE application 102 12 470.1 . In addition, the compounds may also be according to US 6,240,929.
The polyorganosiloxane compounds that contain at least one unit of the formula (I) are, for example, linear polysiloxane copolymers of the general formula (Γ):
-[Q-V]- (I') in which Q is as defined above,
V is at least one V1 group and at least one V2 group,
where V1 and V2 are each as defined above. In addition, V may also be trivalent or higher-valency, particularly trivalent, V3 radicals. In this case, tri- or tetravalent Q units, as defined above, are also present, and the trivalent or higher-valency V3 radicals and the tri- or tetravalent Q units are saturated exclusively by one another within the linear main chain to form cyclic structures, as illustrated in more detail below. However, this case is less preferred.
In a preferred embodiment of the polyamino- and/or polyammonium- polysiloxane copolymers a1 ) of the formulae (I) or ( ), Q is therefore selected from the group consisting of:
-NR-,
-NR+R2- a saturated or unsaturated diamino-functional heterocycle of the formulae:
an aromatic diamino-functional heterocycle of the formula:
in which R is as defined above, and V is selected from V1 and V2.
In the general formulae (I) and ( ), the molar ratio of the V1 and V2 groups in the polysiloxane compounds V2/V1 may in principle assume any value. The invention thus also includes the case in which the polysiloxane compound of the formulae (I) or (Γ) contains only V2 units, i.e. the polysiloxane compound has the formula -[Q-V2]-. The invention also embraces the case in which the polysiloxane compound contains only V1 units. However, in this case, the V1 units have to contain Z1-siloxane units.
In a preferred embodiment of the invention, however, the polysiloxane compound of the formulae (I) or (Γ) contains both V2 and V1 units.
In a further preferred embodiment of the present invention, the molar ratio of the V1 and V2 groups in the polysiloxane compounds of the general formulae (I) and (Γ) is:
V2/V1 = 1
Such linear amine and tetraorganoammonium compounds have been described, for example, in WO 02/10257, WO 02/10259, EP 282720 or US 5,981 ,681. Particular preference is given to the polysiloxanes of WO 02/10259 and WO 02/10257, and reference is made here explicitly to the polysiloxane polymers defined in claim 1 , which form part of the disclosure of the present application.
In a further embodiment of the linear polysiloxane compounds of the formula (I) or (Γ), V2/V1 does not equal 1 ; preferably, V2/V1 is < 1 , more preferably < 0.9; even more preferably, V2/V1 fulfills the relationship
0.0005 < V2/V1 < 0.5, more preferably
0.0005 < V2/V1 < 0.3.
The R group is preferably selected from the R2 groups.
In a preferred embodiment of the invention, Q is a divalent radi is selected in the formulae (I) or (Γ) from the group consisting of:
-NR-,
-NR+R2- a quaternized imidazole unit of the structure
a quaternized pyrazole unit of the structure
a diquaternized piperazine unit of the structure
R 8
—J—
(CH
R 2_ ->Nl I t_ R 3
i . a monoquaternized unit of the structure
_N—
I
(CH2)t R8
and a monoquaternized unit of the structure
R8
-it
I
(CH2)t
R2_^„R3 in which t is from 2 to 10,
R is as defined above, preferably R2, R2 is as defined above, and the definition of R2 and the definition of the above R2 group may be the same or different, R3 has the definition of R2, where R2 and R3 may be the same or different, or R2 and R3 together with the positively charged nitrogen atom form a five- to seven-membered heterocycle, which may optionally have one or more nitrogen,
oxygen and/or sulfur atoms in addition,
R5, R6, R7 may be the same or different and are selected from the group consisting of: H, halogen, hydroxy I group, nitro group, cyano group, thiol group, carboxyl group, alkyl group, monohydroxyalkyl group, polyhydroxyalkyl group, thioalkyl group, cyanoalkyl group, alkoxy group, acyl group, acetyloxy group, cycloalkyl group, aryl group, alkylaryl group, and groups of the -NHRW type in which Rw is H, alkyl group, monohydroxyalkyl group, polyhydroxyalkyl group, acetyl group, ureido group, and in each case two of the adjacent R5, R6 and R7 radicals with the carbon atoms binding them to the heterocycles may form aromatic five- to seven-membered rings, and
R8 has the definition of R2, where R8 and R2 may be the same or different.
In the case that Q is a trivalent radical of the formulae
/ / I
— N or or a tetravalent radical — N— , these radicals in the linear copolymers
\ I
of the formula (Γ), as mentioned above, do not serve to branch the polysiloxane copolymers, but rather these radicals are bonded exclusively to especially trivalent V3 radicals to form cyclic structures which are part of the linear main ample a structural element of the formula:
In a preferred embodiment of the polysiloxane compounds of the formula (I) or ( ) as component a1 ), V2 is a group of the formula
-V2*-Z2-V2*- in which Z2 is as defined above and V2* is a divalent, straight-chain cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 40 carbon atoms and may optionally contain one or more groups selected from -0-, -CONH-, -CONR2- in which R2 is as defined above, -C(O)- and -C(S)-, and the V2* radical may optionally be substituted by one more hydroxyl groups.
In the aforementioned embodiment, the inventive linear polysiloxane copolymer may have the following repeat units:
-[V2*-Z2-V2*-Q]-, preferably together with -[V1-Q]-
V2* is a preferably a divalent straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 16 carbon atoms, may be substituted by one or more groups selected from -0-, -CONH-, -CONR2- in which R2 is as defined above, -C(O)-, -C(S)- and may be substituted by one or more hydroxy I groups. Even more preferably, -V2*- is selected from groups of the formulae:
-(CH2)2-, -(C¾)3- , -(CH2)4-, -(CH2)5-, -(C¾>,
-CH=CHCH2-, -CH=CHCH2CH2-,
-CH2CH2C¾OC(0)CH2-, -CH2CH2CH2OC(0)CH2C¾-,
-CH=CHC¾OC(0)CH2-, -CH-CHCH2OC(0)CH2CH2-,
CH3
-CH2CH2CH2(OCH2CH2)v(OCH2tH)wOC(0)CH2-
CH3
-CH2CH2CH2(OCH2CH2)v(OCH2tH)wOC(0)CH2CH2-
CH3
-CH=CHCH2(OCH2CH2)v(OCH2iH)wOC(0)CH2-
CH3
-CH=CHCH2(OCH2CH2)v(OCH2tH)wOC(G)CH2CH2-
CH3
-CH=CHCH2CH2(OCH2CH2)Y(OCH2tH)wOG(0)CH2-
CH3
-CH=CHCH2CH2(OCH2CH2)v(OCH2tH)wOC(0)CH2CH2-
CH3
-(CH2)l0C(O)(OCH2CH2)v(OCH2tH)wOC(O)CH2CH2-
CH3
-(CH2)l0C(O)(OCH2CH2)v(OCH2tH)wOC(O)CH2- where v+w > 0,
-(CH2)30CH2CHCH2- -(CH2)30C¾CH-
-CH2CH- -CH2CH,
-(C¾)3- , -(CH2)4-, -(C¾)5-3 -(CH2)6-,
-CII-CHCH,-, ~CH=CHCH2CH2-,
-CH2C¾CH2OC(0)CH2-, - CH2CH2CH2OC(0)CH2CH2-., R2 is preferably: H,
-CH3, -CH2CH3, -(CH2)2CH3, -(CH2)3CH3, -(CH2)5CH3, -CH2CH2OH,
where
R4 = straight-chain, cyclic or branched C-\ to Ci8-hydrocarbon radical which may be substituted by one or more groups selected from -0-, -NH-, -C(O)- and -C(S)- and may be substituted by one or more OH groups, especially unsubstituted C5 to Ci7-hydrocarbon radicals which derive from the corresponding fatty acids or else hydroxylated C3 to Ci7-radicals which can be traced back to hydroxy I ated carboxylic acids, especially saccharide carboxylic acids, and quite especially
■ -R9-, in which R9 is a divalent, saturated or mono- or polyunsaturated, straight-chain or branched hydrocarbon radical having from two to 25 carbon atoms,
-(CH2)uC(0)0-[(CH2CH20)q-(CH2CH(CH3)Or]-C(0)(CH2)u
-(CH2)uC(0)0-R9-0-C(0)(CH2)u- in which R9 is as defined above,
■ -(CH2)u-R10-(CH2)u- in which R10 is an aromatic group,
-[CH2CH20]q-[CH2CH(CH3)0]rCH2CH2-,
-CH(CH3)CH20[CH2CH20]q-[CH2CH(CH3)0]r-CH2CH(CH3)- -CH2CH(OH)CH2-,
-CH2CH(OH)(CH2)2CH(OH)CH2-,
-CH2CH(OH)CH2OCH2CH(OH)CH2OCH2CH(OH)CH2- and
-CH2CH(OH)CH20-[CH2CH20]q-[CH2CH(CH3)0]rCH2CH(OH)CH2-
in which
u is from 1 to 3,
q and r are each from 0 to 200, preferably from 0 to 100, more preferably from 0 to 70 and particularly preferably from 0 to 40, and
q + r > 0.
Preferred variants of V1 are structures of the formula:
-CH2C(0)0-[CH2CH20]q-[CH2CH(CH3)0]rC(0)CH2-,
-CH2CH2C(O)0-[CH2CH2O]q-[CH2CH(CH3)0]r-C(0)CH2CH2-3
-CH2CH2CH2C(0)0-[CH2CH20]q-[CH2CH(CH3)0]r-C(0)CH2CH2CH2-, esterified alkylene, alkenylene, alkynylene units, especially of the structures
-C¾C(0)0-[CH2]o-OC(0)CH2-,
-CH2CH2C(0)0-[CH2]o-OC(0)CH2CH2-,
-CH2CH2CH2C(0)0-[CH2]o-OC(0)CH2CH2CH2-
-CH2C(0)0-CH2C=CCH2-OC(0)CH2-,
-CH2CH2C(0)0-CH2G≡CCH2-OC(0)C¾CH2-,
-CH2CH2CH2C(0)0-CH2C≡CCH2-OC(0)CH2CH2CH2-5
-CH2C(0)0-CH2CH=CHC¾-OC(0)CH2-,
-CH2CH2C(0)0-CH2CH=CHCH2-OC(0)CH2CH2-3
-CH2CH2CH2C(0)0-CH2CH=CHCH2-OC(0)CH2CH2CH2-5
alkylene, alkenylene, alkynylene and aryl units, especially of the structures: -[CH2]o- where o = from 2 to 6,
polyalkylene oxide units, especially of the structures
-[CH2CH20]q-[CH2CH(CH3)0] CH2CH ,
-CH(CH3)CH20[CH2CH20]q-[CH2CH(CH3)0]r-CH2CH(CH3)- with
mono-, di- or polyhydroxy-functional units, especially of the structures
-CH2CH(OH)CH2-, -CH2CH(OH)(CH2)2CH(OH)CH2-,
-CH2CH(OH)CH2OCH2CH(OH)CH2OCH2CH(OH)CH2-,
-CH2CH(OH)GH20-[CH2CH20]q-[CH2CH(CH3)0]r-CH2CH(OH)CH2- where
q = from 0 to 200,
r = from 0 to 200.
Preferably, q = from 1 to 50, in particular from 2 to 50, especially from 1 to 20, very especially from 1 to 10, and also 1 or 2, r = from 0 to 100, in particular from 0 to 50, especially from 0 to 20, very especially from 0 to 10, and also 0 or 1 or 2.
The polysiloxanes a1 ) may be prepared as described in the patents above.
The polysiloxanes of the general formula (I) used as component a1 ) in accordance with the invention may also contain branch units V3. V3 is a trivalent or higher-valency, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 1000 carbon atoms and may optionally contain one or more groups selected from -0-, -CONH-, -CONR2- where R2 is as defined above, -C(O)-, -C(S)-, -Z1-, which is as defined above, -Z2- which is as defined above and Z3 where Z3 is a trivalent or higher-valency polyorganosiloxane unit. The branch unit V3 may be silicone-free. Examples thereof include:
where v + w > 0.
The branch unit V3 may be a trivalent or high-valency polyorganosiloxane unit, for example:
in which R is in each case as defined above.
One example of a Z -containing branch unit V3 is, for example:
The polyamino- and/or polyammonium-polysiloxane compounds a1 ) used may be solid or liquid at 25 °C. In the case that they are liquid at 25 °C, the viscosities of the polysiloxanes a1 ) mentioned are preferably between 500 and 50 000 000 mPa.s at 25 °C, preferably from 1000 to 2 500 000 mPa.s at 25 °C, and at a shear rate gradient of D = 1 s"1. They may have melting points up to 250 °C, but are water-soluble or -dispersible. Their solubility is preferably more than 1 g/l at 25°C. The preferred concentration of amino or ammonium units are in the range of 2 to 4 amino or ammonium units per 50 -100 organosiloxy units.
The component a) used may be preferably one or more one amino- and/or ammonium-polysiloxane compounds a2). As explained above, these
contain amino or ammonium groups only in the pendant groups. The amino- and/or ammonium-polysiloxane compounds a2) are preferably polysiloxanes which bear primary and/or secondary and/or tertiary amino groups in the pendent groups, in which the amino groups may optionally be protonated or quaternized, and which may optionally contain additional hydrophilic groups. The amino or ammonium groups mentioned are preferably bonded to the siloxane skeleton via carbon. The amino- and/or ammonium-polysiloxane compounds a2) mentioned are preferably polyalkylsiloxanes having aminoalkyl- or aminoarylsiloxane units. The aminoalkyl units may be bonded to the difunctional, trifunctional or the monofunctional end groups, and be part of other oxygen-containing pendent groups, in particular of polyether pendent groups.
The additional hydrophilicizing groups optionally present are preferably those, which derive from polyalkylene oxides and saccharides.
The aminopolysiloxanes mentioned are linear or branched polysiloxanes which are formed from siloxy units which are selected from the group consisting of:
(Q) CO Φ) (M) in which R11 represents organic radicals which may be the same or different with the proviso that at least one of the R11 radicals contains at least one nitrogen atom.
The R11 substituents are preferably selected from the group consisting of:
straight chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 200 carbon atoms and may
:ionally contain one or more groups selected from:
-0-,
-NR2- where R2 may be hydrogen, a monovalent, straight-chain, cyclic or branched, saturated, unsaturated or aromatic hydrocarbon radical which has up to 100 carbon atoms, may contain one or more groups selected from -0-, -NH-, -C(O)- and -C(S)-, and is optionally substituted by one or more substituents selected from the group consisting of a hydroxyl group, an optionally substituted heterocyclic group preferably containing one or more nitrogen atoms, amino, alkylamino, dialkylamino, ammonium, polyether radicals and polyether ester radicals, where, when a plurality of -NR2 groups is present, they may be the same or different,
— —
-C(O)- and -C(S)-, and the radical may optionally be substituted by one or more substituents selected from hydroxyl and
where R1 , m, m2 are each as defined above, hydroxy I,
a polyether radical which has up to 20 000 carbon atoms and may optionally bear one or more amino, mono- or dialkylamino, or arylamino groups,
a saccharide-containing organic radical,
or two R1 1 substituents from different siloxy units together form a straight- chain, branched or cyclic alkanediyi radical having from 2 to 12 carbon atoms between two silicon atoms,
with the proviso that at least one R1 1 substituent per molecule contains nitrogen, i.e., constitutes a nitrogen-containing R11 radical.
R11 is preferably alkyl, in particular methyl.
Preferred R1 1 radicals, which have nitrogen are, for example:
ΟΗ
I
-CH2CH2CH2NH2 -CH2CH2CH2NHCH2CH2NH2 -CH2CH2CH2OCH2CHCH2NH2
OH OH
-CH2CH2CH2OCH2CHCH2NHCH2CH2NH2 -CH2CH2CH2OCH2CHCH2 HCH2CH2CH2NH2
OH
-CH2CH2CH2OCH2CHCH2N -CH2CH2CH2OCH2CHCH2N O
OH CH2CH2N(CH3)2 OH CH2CH2CH2N(CH3)2
-CH2CH2CH2OCH2CHCH2 N -CH2CH2CH2OCH2CHCH2N
CH2CH2N(CH3)2 CH2CH2CH2N(CH3)2
Further preferred amino- and ammonium-containing R11 radicals are, for example:
Corresponding aminopolysiloxanes having such R 1 radicals are disclosed in WO 02/10256, whose disclosure content belongs to the present application. The nitrogen-containing R1 1 radical is preferably aminopropyl or aminoethyl- aminopropyl. Further preferred nitrogen-containing R1 1 radicals are formed from the reaction of glycidyloxypropylsiioxanes with mono- or dialkylamines. Preferred compounds as component a2) are therefore, for example, aminopolysiloxanes which arise from the reaction of epoxyalkylsiloxanes with ammonia, primary or secondary amines, such as those from the reaction of glycidyloxypropylsiioxanes with mono- or dialkylamines. Preferred alkoxy radicals for R1 1 are methoxy, ethoxy, propoxy, isopropoxy, butoxy, hexyloxy and cyclohexyloxy.
Preferred polyether radicals which have up to 20 000 carbon atoms and may optionally bear one or more amino-, mono- or dialkylamino, or arylamino groups for R1 1 include, for example:
CH3
-CH2CH2CH2(OCH2CH2)v(OCIl2CH)wOH
CH3
-CIl2CH2CH2(OCH2CH2)v(OCH2CH)wOC(0)CH3
CH3
-CH2CH2CH2(OCH2CH2)v(OCH2CH)wOCH3
CH3
-CH-CHCH2(OCH2CH2)v(OCIl2CH)wOH
CH3
-CH=CHCH2(OCH2CH2)v(OCH2CH)wOC(0)CH3
CH3
-CH=CHCH2(OCH2CH2)v(OCH2CH)wOCH3
CH3
-CH-CHCH2CH2(OCH2CH2)v( CIl2CH)wOH
CH3
-CH=CHCIi2CH2(OCIl2CH2)v(OCH2CH)wOC(0)CIl3
CH3
-CH=CHCIl2CH2(OCI-l2CH2)v(OCH2CII)wOCH3
Saccharide-containing organic radicals for R1 1 are, for example:
The preparation of such saccharide-containing polysiloxanes can be
found, for example, in DE 4 318 536, DE 4 318 537.
The average degree of polymerization of the polysiloxane moiety of the aminopolysiloxanes a2) used in accordance with the invention, which results from Mn, is appropriately from 1 to 3000, preferably from 50 to 1000.
The typical content of polar groups in the polyorganosiloxanes a2) is between 0.03 % by weight to 3 % by weight. The preferred polar group in a2) are amino groups, wherein the typical nitrogen content of such aminopolyorgano- siloxanes a2) used in accordance with the invention is, for example, between 0.03 % by weight to 3 % by weight, preferably from 0.3 % by weight to 1.3 % by weight, more preferably from 0.4% by weight to 1.2 % by weight. This relates to aminopolyorganosiloxanes a2), wherein polyorganosiloxanes are polydimethyl- siloxanes. The values for the nitrogen concentration have to be adjusted for polyorganosiloxanes having other groups than methyl.
The aminopolysiloxanes a2) used may be solid or liquid at 25 °C. In the case that they are liquid at 25 °C, the viscosities of the aminopolysiloxanes a2) used in accordance with the invention are preferably between 50 to 100 000 mPa.s at 25 °C, preferably from 100 to 3 000 mPa.s at 25 °C, and at a shear rate gradient of D = 1 s'
They may have melting points up to 250 °C, but are water-soluble or -dispersible. Their solubility is preferably more than 1 g/l at 25 °C.
Some of the above-described aminopolysiloxanes a2) used in accordance with the invention are obtainable, for example, as Wacker Finish* WR 1 100 and Momentive® SF 1923.
Preferably the compositions according to the invention contain component a2).
The polyorganosiloxanes containing polar groups of the present invention are preferably any polysiloxanes having at least one Si-C bonded polar organic group selected from the group consisting of amino, ammonium, epoxide, hydroxy, polyhydroxy, mercapto, or carboxyl and sulfonic acids, their esters and salts. Preferred are polyorganosiloxanes containing Si-C bound pendant amino
or ammonium, hydroxy I or carboxyl acid polar groups. Especially preferred are polyorganosiloxanes with at least 0.3 mmol/g pendant polar groups.
Most preferred are polyorganosiloxanes of the general structure:
M-Dx-D*y-M
Wherein:
M = (CH3)3Si-01 /2- or R*(CH3)2Si-Oi /2- D = -(CH3)2Si-0- D* = -R*CH3Si-0-
R* = -(CH2)n-Q group (n = 1 -3, preferably n is 3) and Q comprises one or more of the polar groups such as preferably
-NH2 ,
-NH-CH2CH2-NH2 l
-NH-CH2CH2-NH-E, wherein E is CrC22-alkyl, preferably methyl, aryl, preferably phenyl,
-NH-CH2CH2-NH-C(OHCH2)3-C02 H,
-NH-C(0)-C6 H5,
-OCH2-C(C2 H5)(CH2OH)2 ,
-0-CH2-CH(OH)-CH2OH or
with y > 1 , and x + y < 1000, wherein x and y represent the average degree of polymerization.
Preferably the component a2) contains at least 0.3 mmol/g of the polar groups, in particular, the amino and/or ammonium groups. Still more preferably the component a2) contains between 0.3 and 0.9 mmol/g of the polar groups, in particular, the amino and/or ammonium groups. Particularly preferred is the range of 0.35 to 0.6 mmol/g in particular of the amino and/or ammonium groups. These concentrations apply to polydimethylsiloxane compounds. The value 0.3 mmol/g corresponds approximately to 2.2 polar groups per 100 dimethylsiloxy groups. These compounds may contain traces of alkoxysilyl groups coming out of an uncompleted condensation reaction of the precursors.
As the component b) the composition according to the invention contains more than 50 to 150 parts by weight (pw), preferably >50 to 120 pw of one or more alkylpolyglucosides (sometimes referred to as APG), based on 100 parts of component a). Still more preferably the weight ratio of the alkylpolyglucosides b) to the polyorganosiloxanes a) is less than 1 or the amount of the alkylpolyglucosides b) is less than 100 pw based on 100 pw of component a). Preferably the weight ratio of the APGs to the component a) is between 0.5 to 1.1 , preferably between 0.5 and 1 .0.
The alkylpolyglucosides b) are preferably at least one compound selected from the group of the following formula (II)
AO-[G]p (II) wherein the unit 'G' is a substituted or non-substituted, preferably non- substituted sugar unit, respectively, containing preferably 5 or 6 carbon atoms and the index "p' is a number of 1 to 10, and 'A' is preferably selected from the group of straight- or branched-chain C4 - C24-alkyl or alkenyl groups. The sugar unit is selected from the group of aldoses and ketoses, i.e. furanoses and pyranoses, preferably glucoses. In principle, it is possible to convert known polysaccharides, oligosaccharides and monosaccharides such as starch,
maltodextrin, dextrose, galactose, mannose and xylose into alkylpolyglycosides. Industrially accessible carbohydrates such as starch, maltodextrin and dextrose are preferred as precursors. Since the industrially relevant alkylpolyglycoside syntheses are not directed to the selection of regioselectively or stereoselectively preferred derivatives, the alkylpolyglycolsides are in majority mixtures of monomers and oligomers which in turn are mixtures of different isomeric structures. Pyranose and furanose structures are present side by side and have both alpha- and beta-glycosidic bonds. According to the stereochemistry of carbohydrates the position of bonds between pairs of saccharide units can differ. Depending on the method of synthesis, the alkylpolyglycosides can also contain residues of associated substances such as alcohols, monosaccharides, oligosaccharides and oligoalkylpolyglycosides.
The formula (II) therefore represents all types of carbohydrates, monomers and polymers including furanoses and pyranoses, such as
A -[C6Hio05]pH .(Ila) or
A -[C5H804]PH (lib)
Individual examples for the generic formulas (Ila) and (lib) are e.g. expanded structures such as:
(Ila)
A (for the alkylpolyglycoside formulas) is selected from the group of straight- or branched-chain, C4-C24-alkyl or alkenyl groups preferably C8-C24-alkyl or alkenyl groups or a mixture of straight- or branched-chain, C4-C24-alkyl or alkenyl groups preferably C8-C24 -alkyl or alkenyl groups;
Z is selected from the group of hydrogen, C1-C4-alkyl or alkenyl groups or a mixture of straight- or branched-chain, C C4-alkyl or alkenyl groups;
-P(0)(OR3 )2 , -N02> -S(0)2(OR31), -C(0)-R31 ; -C(0)0-R31 , -CNH-R31 groups, R31 is selected from the group of hydrogen, C - C10-alkyl or alkenyl groups or a mixture of straight- or branched-chain, Ci - C8-alkyl or alkenyl groups.
The alkyl or alkenyl radical A may be preferably derived from compounds such as primary alcohols containing 4 to 22 and preferably 8 to 18 carbon atoms. Typical examples are butanol, pentanol, caproic alcohol, caprylic alcohol, capric alcohol and undecyl alcohol and the technical mixtures thereof obtained, for example, in the hydrogenation of technical fatty acid, fatty acid derivatives, e.g. methyl esters, Ziegler alcohols or in the hydrogenation of aldehydes from 'Roelen's Oxo synthesis'. Further alcohols include lauryl alcohol, myristyl alcohol, cetyl alcohol, palmitoleyl alcohol, stearyl alcohol, isostearyl alcohol, oleyl alcohol, elaidyl alcohol, petroselinyl alcohol, arachyl alcohol, gadoleyl alcohol, behenyl alcohol,
erucyl alcohol, brassidyl alcohol and technical mixtures thereof which may be obtained as described above.
The index 'p' in the general formula (II) indicates the degree of polymerization (Pn) calculated on the basis of average number of molecular weight, i.e. represents the distribution of mono- and oligoglycosides. The index 'p' is a number from 1 to 15.
Although the degree of polymerization Pn or index p' in an individual compound is an integer and, above all, may have a preferred value of 1 to 6, the value 'p' for a commercial alkyl oligoglycoside is an analytically determined calculated value which as a result is generally a fractional number. Alkyl and/or alkenyl oligoglycosides having an average degree of oligomerization 'p' of 1.1 to 3.0 are preferably used. Alkyl and/or alkenyl oligo-glycosides, which have a degree of polymerization of less than 1 .7 and, more particularly, between 1 .2 and 1 .4, are still more preferred.
The alkylpolyglycosides used in this invention can be prepared partly or entirely on the basis of renewable raw materials by known synthesis. For example, poly- or oligosaccarides are reacted in the presence of an acidic catalyst with n- pentanol to form pentyl oligoglycoside mixtures which are then converted to the desired alkyloligoglycosides with long-chain alcohols in the presence of an acidic catalyst. Usually a composition of several compounds having a distribution regarding the glycosidic and the alkyl component is obtained and used respectively.
Another synthetic approach to the alkylpolygylcosides is to obtain fatty alcohols or mixtures thereof from natural fatty esters, like coconut oil, by transesterification, hydrogenation and subsequent distillation, and to react them with glucose or other hydrolysis products from starch.
The alkylpolygylcosides of formula (II) defined above, used in this invention, are preferably represented by the products sold by the Companies Henkel, Sasol and Cognis under the name APG, such as the products APG 300, APG 350, APG 500, APG 550, APG 625, APG base 10-12, Plantacare® or Glucopon®, the products sold by the company BASF under the name LUTENSOL® GD 70 e.g., sold by Seppic under the names TRITON® CG 110 (or ORAMIX© CG 1 10) and TRITON® CG 312 (or ORAMIXNS 10).
The preferred alkylpolyglycosides of formula (II), according to the invention, are APG 300 of the Company Henkel, TRITON® CG 1 10 of the Company Seppic and LUTENSOL® GD 70 of the Company BASF, especially preferred Plantacare® 2000 and Glucopon® 600 or 215.
Alkyloligoglucosides based on hydrogenated C12/C14-coconut alcohol with a Pn of 1 to 3 are preferred.
Alkyloligoglucosides having a alkyl chain length of C8 - C12 (Pn=1 to 3), which are obtained as first runnings in the separation of technical C8- C18 coconut oil fatty alcohol by distillation and which may contain less than 6 wt.% of C12 alcohol as remaining impurity, and also alkyloligoglucosides based on technical C9/C1 1 - oxoalcohols (Pn =1 to 3) are especially preferred.
Alkylpolyglycosides are described for example in DE-A-1 943 689, EP-A-418479 or EP-A-1972817.
The aqueous emulsions according to the present invention comprise one or more ionic co-surfactants c). Ionic surfactants include surfactants that have ionic or charged groups, like ammonium, phosphonium, sulfate, carboxylate, sulfonate, phosphonate, and suitable counter ion or counter ionic groups, including metal or non-metal cations, acid anions, like halogen anions, acetate, tosylate, sulfate, phosphate, nitrate etc.
Component c) include one or more co-surfactants selected from the group consisting of amphoteric or zwitterionic surfactants, amine oxide surfactants and cationic surfactants.
In a preferred embodiment those co-surfactants c) are selected from amphoteric or zwitterionic and cationic surfactants, having preferably more than 6 carbon atoms.
Cationic surfactants include for example nonpolymerized, organic, quaternary ammonium compounds, preferably hydrocarbon-containing quaternary ammonium salts or amine salts, where the hydrocarbon groups may preferably contain from 8 to 28 carbon atoms.
Examples of components c) are compounds of the following formula:
R12R13R14R15N +X- in which R12, R13, R14 and R15 are each independently selected from the group consisting of: C C28-alkyl, alkenyl, hydroxyalkyl, benzyl, alkylbenzyl, alkenylbenzyl, benzylalkyl and benzylalkenyl, and X is an anion. The R12, R13, R14 and R15 groups may each independently contain one or more, preferably two, ester (-[-O-C(O)-]; [-C(0)-0-]) and/or amido groups (~[CO-N(R12)-]; [-N(R12)-CO-]) in which R12 is as defined above. The anion X may be selected from halides, methosulfate, acetate and phosphate, preferably from halides and methosulfate. Further examples of component c) are tetraorgano-substituted quaternary ammonium compounds having one or two long-chain C8 to C28 hydrocarbon radicals and two or three short-chain C1 to C6 hydrocarbon radicals. The long- chain radicals are preferably C12 to C20 chains and the short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and hydroxyethyl, 2-hydroxypropyl. Preferred counterions are CI", Br", CH3OS03 ~, C2H5OS03\ N03 ", HCOCr and CH3COO\
Examples include:
dodecylethyldimethylammonium bromide
didodecyldimethylammonium bromide.
In the cationic surfactants, which contain only one long-chain hydrocarbon group R 2, the chain length of the long-chain hydrocarbon group is preferably from 12 to 15 carbon atoms, and the short-chain radicals R13, R14 and R15 are preferably methyl and hydroxyethyl.
In the cationic surfactants, which contain two, three or even four long-chain hydrocarbon groups, the chain length of the long-chain hydrocarbon groups is preferably from 12 to 28 carbon atoms.
Preferred ester-containing surfactants have the formula:
{(R16)2N[(CH2)ZER17]2}+X- in which R16 is independently selected from Ci-4 alkyl, hydroxyalkyl or C2-4 alkenyl; and in which R17 is independently selected from Cs-28 alkyl or alkenyl groups; E is an ester group, i.e. -OC(O)- or -C(0)0-, z is an integer from 0 to 8 and X" is as defined above. They contain two or three short-chain C1 to C6 hydrocarbon radicals. The one or two long alkyl radicals per molecule derive from fatty acids of lengths from C8 to C26, preferably from C10 to C20, especially from C12 to C18. The fatty acids or the cuts of the chain length ranges mentioned may be saturated fatty acids, unsaturated fatty acids, hydroxy-substituted fatty acids or mixtures thereof. Examples of the acids mentioned are lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and ricinoleic acid. Further examples are tallow fatty acid and coconut fatty acid cuts. They are bonded to the quater- nized nitrogen preferably via oxyethyl, 2-oxypropyl or 1 ,2-dioxypropyl or oligooxyethylene spacers. The short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and hydroxyethyl, 2-hydroxypropyl. Preferred counterions are CI", Br", CH3OS03 ", C2H5OS03 ", N03 ", HCOO" and CH3COO". Examples include
(tallow fatty acid oxyethyl)trimethylammonium methosulfate
(coconut fatty acid pentaethoxy)trimethylammonium methosulfate
di(tallow fatty acid oxyethyl)dimethylammonium chloride
di(tallow fatty acid oxyethyl)hydroxyethylmethylammonium methosulfate di(tallow fatty acid 2-oxypropyl)dimethylammonium methosulfate
1 ,2-(ditallow fatty acid)oxy-3-trimethylpropaneammonium chloride
A further type of preferred ester-containing cationic surfactants can be represented by the following formula:
{(R16)3N(CH2)ZCH [0(0)CHR17] [CH20(0)CR17]}% in which R16, R17, X, and z are each as defined above.
A further group of cationic surfactants c) is those of the formula R18A(CH2)2-4NR19R20 in which R18 is C6-C12 alkyl;
A is a divalent group which is selected from -NH-, -CONH-, -COO- or -0-, or A may be absent. R19 and R20 are each independently selected from the group consisting of H, C Ci4 alkyl or (CH2-CH2-0(R21)) in which R21 is H or methyl.
Particularly preferred surfactants of this type are decylamine, dodecylamine, C8-C12 bis(hydroxyethyl)amine, C8-Ci2 bis(hydroxypropyl)amine, C8-C 2 amidopropyldimethylamine or salts thereof.
Further surfactants include: fatty acid amides of the formula R 2C(0)N(R23)2 wherein R22 is an alkyl group having from 8 to 28 carbon atoms and R23 is in each case a short-chain radical, preferably selected from hydrogen, Ci-C6 alkyl and hydroxyalkyl. It is also possible to use C8-C28 N-alkylpolyhydroxy fatty acid amides. Typical examples include: C12-C18 N-methylglucamides (see WO 92/06154). Other sugar derivatives include, for example, C8-C28 N-(3-methoxypropyl)glucamide. These likewise have two or three short-chain C-i to Ce hydrocarbon radicals. The one or two long alkyl radicals per molecule derive from fatty acids of lengths C8 to C26, preferably C10 to C2o, especially C12 to Ci8. The fatty acids or the cuts of the chain length ranges mentioned may likewise be saturated fatty acids, unsaturated fatty acids, hydroxy-substituted fatty acids or mixtures thereof. Examples of the acids mentioned are lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and ricinoleic acid. Further examples are tallow fatty acid and coconut fatty acid cuts. They are bonded to
the quaternized nitrogen preferably via amidoethyl and 3-amidopropyl spacers. The short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and hydroxyethyl, 2-hydroxypropyl. They may also alternatively be cyclic radicals such as imidazolinium radicals into which fatty alkyl substituents have optionally additionally been incorporated. Preferred counterions are CI", Br", CH3OSO3 , C2H5OSO3", N03 ", HCOO" and CH3COO".
Examples include
(undecylenic acid amidopropyl)trimethylammonium methosulfate
(ricinoleic acid amidopropyl)trimethylammonium methosulfate
1 - methyl-1 -(tallow fatty acid amidoethyl)-2-(tallow fatty alkyl)imidazolinium methosulfate
1 -methyl-1 -oleylamidoethyl-2-oleylimidazolinium methosulfate
1 , 1 -ethylenebis(1 -methyl-2-(tallow fatty alkyl)imidazolinium) methosulfate.
In addition to the quaternary ammonium compounds, amine salts may also find use. These are salts of primary, secondary or tertiary amines with inorganic or organic acids.
In these amine salts, the nitrogen is substituted by one or two long- chain C8 to C28 hydrocarbon radicals, one to three hydrogen atoms and optionally one or two short-chain Ci to C6 hydrocarbon radicals. The one or two long alkyl radicals per molecule derive, for example, from fatty amines or fatty acids of lengths C8 to C2e, preferably Ci0 to C20, especially C12 to Cis atoms. Preferred counterions are CI", Br", CH3OSO3", C2H5OS03 ", N03 ", HCOO" and CH3COO".
The fatty acids or the cuts of the chain length ranges mentioned may be the saturated fatty acids, unsaturated fatty acids, hydroxy-substituted fatty acids or mixtures thereof, which have already been described. Examples of the acids mentioned are lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and ricinoleic acid. Further examples are tallow fatty acid and coconut fatty acid cuts. They are bonded to the amine salt nitrogen preferably via oxyethyl,
2- oxypropyl, 1 ,2-dioxypropyl spacers in the case of esters, and preferably via amidoethyl and 3-amidopropyl spacers in the case of amides. The short-chain radicals are preferably methyl, ethyl, propyl, butyl, hexyl, phenyl and
hydroxyethyl, 2-hydroxypropyl. Preferred counterions are CI", Br", CH3OSO3", C2H5OS03 ", N03 ", HCOO" and CH3COO".
Examples include
stearic acid triethanolamine derivative:
CH3(CH2)16C(0)OCH2CH2N+(CH2CHOH)2 CI"
stearamide derivative CH3(CH2)i6CONHCH2CH2N+H2CH2CH2 +H3 2CI" stearamide derivative CH3(CH2)i6CONHCH2CH2N CH2CH2OH CI"
palmitamide derivative CH3(CH2)14CONHCH2CH2CH2 +H(CH3)2 CI".
Suitable amphoteric or zwitterionic surfactants are described for example in US 5, 104,646 , 5, 106,609 . They include those surfactants broadly described as derivatives of aliphatic secondary and tertiary amines in which the aliphatic radical can be straight or branched chain and wherein one of the aliphatic substituents contains from 8 to 18 carbon atoms and one contains an anionic group such as carboxy, sulfonate, sulfate, phosphate, or phosphonate. Suitable amphoteric surfactants for use in the present invention include cocoampho- acetate, cocoamphodiacetate, lauroamphoacetate, lauroamphodiacetate, and mixtures thereof. Further zwitterionic surfactants suitable for use in the compositions are well known in the art, and include those surfactants broadly described as derivatives of aliphatic quaternary ammonium, phosphonium, and sulfonium compounds, in which the aliphatic radicals can be straight or branched chain, and wherein one of the aliphatic substituents contains from 8 to 18 carbon atoms and one contains an anionic group such as carboxy, sulfonate, sulfate, phosphate or phosphonate. Further zwitterionic surfactants include betaines, and furthermore, although less preferred: amine oxide surfactants like Ci2-Ci4.alkyldimethyl amine oxide.
Non-limiting examples of other ionic additional surfactants suitable for use in the compositions are described in McCutcheon's, Emulsifiers and Detergents, 1989 Annual, published by M. C. Publishing Co., and US 3,929,678, US 2,658,072; US 2,438,091 ; US 2,528,378.
The co-surfactants used in the aqueous emulsions according to the invention are preferably essentially free of (poly)oxyalkylene groups.
Preferably the entire aqueous emulsions according to the invention are essen tially free of (poly)oxyalkylene compounds, that is, compounds that have two or more oxyalkylene groups. Essentially free means that the aqueous emulsions contain less than 1 % by weight more preferably less than 0.5 % by weight based on the entire emulsion.
In a preferred embodiment of the invention the weight ratio of the ionic co-surfactants to the alkyl polyglucosides is between 0.001 and 0.5. Preferably the aqueous emulsions according to invention comprise 0.1 - 50 parts of one or more ionic co-surfactants, based on 100 pw of the polyorganosiloxanes.
The preferred co-surfactants of the instant invention may include ionic organic surfactants that contain at least one Ci0 - C30-alkyl or alkylaryl group and at least one ionic hydrophilic group. Preferred are cosurfactants that have cationic, amphoteric, zwitterionic or (less preferred) amine oxide structures. Most preferred are cosurfactants that do not contain any oxyalkylene groups. Particularly preferred examples of cationic surfactants include quaternary ammonium compounds and their salts, such as tallowtrimethylammonium chloride, dodecyltrimethyl ammonium chloride, hexadecyltrimethylammonium chloride, didodecyldimethylammonium chloride, dicocoalkyldimethylammonium chloride, n-alkyldimethylbenzeneammonium chlorides, as well as the corresponding hydroxides, acetates or fatty acid salts. Other examples are the primary, secondary or tertiary aliphatic amine salts such as dodecylammonium acetate, alkyldimethylammonium chloride or the like. Preferred are alkyltrimethylammnium chlorides or dialkyldimethylammonium chlorides, such as hexadecyltrimethylammonium chlordie and dicocoalkyldimethylammonium chloride, or the corresponding hydroxides or acetates.
Preferred examples of zwitterionic surfactants are amino acid based surfactants and related betaine surfactants, preferred examples of which are cocoalkylamido- propylbetaine, N-lauryldimethylbetaine, cocoalkylamidopropylsulfobetaine, 3- (dodecyldimethylamonio)-propansulfonate and the like.
Especially preferred is cocamidopropyl betaine of the formula:
Component c) includes also amine oxides. Suitable amine oxide surfactants have tho fnrmi ιΙα·
R^sN-O, wherein R is identical or different, and is a straight or branched optionally substituted alkyl group, wherein one or more methylene groups might be replaced by O, N H , and optional substituents may include 1 to 3 substituents, including hydroxyl etc.
Non-limiting examples of suitable amine oxide compounds include dimethyl- dodecylamine oxide, oleyldi(2-hydroxyethyl) amine oxide, dimethyltetradecyl- amine oxide, di(2-hydroxyethyl)-tetradecylamine oxide, dimethylhexadecylamine oxide, behenamine oxide, cocamine oxide, decyltetradecylamine oxide, dihydroxyethyl Ci2-Ci5-alkoxypropylamine oxide, dihydroxyethyl cocamine oxide, dihydroxyethyl lauramine oxide, dihydroxyethyl stearamine oxide, dihydroxyethyl tallowamine oxide, hydrogenated palm kernel amine oxide, hydrogenated tallowamine oxide, hydroxyethyl hydroxypropyl Ci2-Ci5-alkoxypropylamine oxide, lauramine oxide, myristamine oxide, myristyl/cetyl amine oxide, oleamidopropyl-
amine oxide, oleamine oxide, palmitamine oxide, , dimethyl lauramine oxide, potassium trisphosphonomethylamine oxide, stearamine oxide, and tallowamine oxide. In various embodiments, the amine oxide is a dimethyl lauramine oxide.
Particularly preferred examples of amine oxides are alkylamine oxides or alkyldimethylamine oxides, such as lauryldimethylamine oxide, stearyldimethyl- amine oxide or cocosalkyldimethylamine oxide and the like.
The aqueous emulsions according to invention comprise 100 - 1000 parts by weight preferably 150 to 800 parts by weight, more preferably 200 to 600 parts by weight of water based on 100 parts by weight of the component a), i.e. the polyorganosiloxanes.
Due to the specific composition of the aqueous emulsions according to the invention, they are usually optically clear.
In the aqueous emulsions according to the invention usually the components a) to c) are finely divided as droplets in the aqueous phase. Usually the droplets have a volume average d50 size of less than 120 nm, preferably less than
100 nm, measured by laser scattering method preferably performed by using a Beckman Coulter Particle Sizer Model LS 13320 preferably according to the Mie- Theory of light scattering applying the method ISO 13320-1 (1990): Particle size analysis - Laser diffraction methods. See also I. Zimmermann Ingenieur Technik 68 (1996) Nr. 4.
Additionally, the aqueous emulsions according to the invention may contain water soluble compounds such as glycerine, low molecular weight alcohols, organic or inorganic salts, amines and the like. Additionally, these emulsions may contain additives such as biocides, perfumes, dies, pigments, thickeners or fillers. Preferred are emulsions that contain less than 5 % by weight such additives. Most preferred are emulsions should contain less than 3 % by weight additives and less than 1 % by weight total biocides.
Additionally, the pH of the emulsions may be controlled using common acids, bases, like Methanol amine, common amino acids and the corresponding buffers.
The microemulsions according to the invention form spontaneously upon mixing without the use of high shear and are characterized by an average particle size of preferably less than 120 nanometers causing the emulsions to be optically clear. These inventive microemulsions are stable to dilution with water to less than 2 parts polyorganosiloxane in 100 parts of water, without phase separation.
These inventive aqueous emulsions may also comprise other oil soluble compounds such as paraffin oil, non-polar cyclic siloxanes or polyorganosiloxanes, or organic alcohols, amines, esters or ketones. Preferred are emulsions that comprise oil phases containing at least 20 % by weight of one or more polyorganosiloxanes a) containing polar pendant groups.
The aqueous emulsions according to the invention can be used in particular for the manufacture of cosmetic compositions, in particular also for transparent cosmetic compositions, for example for the manufacture of hair care compositions, such hair shampoos, hair gels, hair conditioners, skin care compositions such skin cleansing compositions, skin cleansing gels, carrier such as ointment basis for bioactive ingredients such as cosmetically or pharmaceutically active ingredients, carrier for chemicals in general such as active ingredients, such as pharmaceuticals, agrochemicals such as herbicides, insecticides, fungicides etc. Furthermore, they can be used for treatment of fibers or fibrous materials, such as initial textile treatment, in particular to provide antistatic and/or softening properties to textile fibers, paper or paper tissue, non- wovens, leather or furs. The inventive emulsions can be used in the treatment of carbon or glass fibers or substrates. Furthermore, the inventive emulsions can be used in the treatment of painted surfaces, glass or plastic surfaces, for examples car bodies or windshields, rendering these surfaces more hydrophobic and/or more resistant to weathering and cleansing or polishing agents. Furthermore, the inventive emulsions can be used alone or formulated with other materials in the
treatment of materials used in construction and civil engineering where water repellency is desired. This includes materials such as masonry and wood products, such as brick, paving materials, asphalt, cement, plaster, molding, roofing tiles, stucco, porcelain, stone, marble, tiles, adobe, concrete, masonite, mineral board, particle board, gypsum and so forth. The treated construction materials are characterized by improved water repellency or water-proofness and/or improved resistance to weathering. Furthermore, the aqueous emulsions can be used for surface treatment, for example in cleansing, polishing or disinfection compositions. In all these applications it is an advantage of the aqueous emulsions that they are optically clear or transparent. The aqueous emulsions according to the invention are preferably optically clear 'oil-in-water' emulsions
EXAMPLES Example 1
Microemulsion of Siloxane (1), alkylpolyglycoside and a cationic cosurfactant with an APG/Siloxane ratio of 0.83
20 g of a 62 % actives solution of an alkylpolyglycoside (available as Glucopon 215 UP from the Cognis GmbH), 15 g of a aminopolysiloxane with pendant polar groups consisting of dimethylsiloxy, methyl(N-[2-aminoethyl]-3-aminopropyl)- siloxy units and trimethylsiloxy endcapping groups and with a viscosity of 1015 mPas*s at 25 X and 0.45 mmol/g of amino groups, 4 g of a 75 % actives solution of Dicocoalkyldimethylammonium chloride (Arquad 2C-75 available from Akzo Nobel), and 56.8 g of deionized water were mixed at room temperature. The mixture was acidified to a pH value of 5 with 1.2 g of acetic acid and heated to 70 °C for 30 minutes, resulting in a clear microemulsion. Neutralisation with 2,0 g of a 60 % triethanolamine solution gave a final pH value of 8. Finally 1 ,0 g of a 30 % actives solution of parabenes in 2-phenoxyethanol was added as a preservative. The resulting product was a optically clear emulsion with an average particle size of 65 nm, a silicone content of 20 wt.-%, a total solids content of
34.2 wt.-%, a viscosity at 23 °C of 4,3 mPa*s. After 5 weeks at 40 °C the emulsion was unchanged. Diluting the emulsion 5:2 with a 10 % sodium chloride solution gave no change. The emulsion was also stable to dilution with a 10 % solution of sodium carbonate.
Comparative Example 1
Emulsion of Siloxane (1 ) and alkylpolyglycoside with an APG/Siloxane ratio of 0.83 without co-surfactant
20 g of a 62 % actives solution of an alkylpolyglycosides (Glucopon 215 UP),
15 g of the aminopolysiloxane from Example 1 and 64 g water were mixed at room temperature. The mixture was acidified to a pH value of 5 with 1 .2 g of acetic acid and heated to 70 °C for 60 minutes. The emulsion remained opaque. Neutralization of a portion of the emulsion to pH of 8 with a 10 % solution of sodium carbonate caused the emulsion to break. Neutralization with 2.0 g of a 60 % triethanolamine solution gave a final pH value of 7 resulted in a stable emulsion with an average particle size of 288 nm, a total solids content of
31 .43 % and a viscosity at 23 °C of 5.9 mPa*s. Diluting the emulsion 5:2 with a 10 % sodium chloride solution caused the emulsion to break and separate into two phases.
Example 2
Microemulsion of Siloxane (2), alkylpolyglycoside and a zwitterionic cosurfactant with an APG/Siloxane ratio of 0.75
30 g of a 50 % actives solution of alkylpolyglycosides (available as Plantacare 2000 UP from Cognis Carechemicals), 20 g of a aminopolysiloxane with pendant polar groups, consisting of dimethylsiloxy, methyl(N-[2-aminoethyl]-3-amino- propyl)siloxy units and thmethylsiloxy endcapping groups, with a viscosity of 1960 mPas*s at 25 °C and 0.77 mmol/g of amino groups, 4 g of a 33 % actives solution of cocoamidopropyl betaine (available as Amonyl 380 BA from SEPPIC) and 42 g of deionized water were mixed at room temperature. The mixture was acidified to a pH value of 5 with 1 .3 g of acetic acid, resulting in a clear microemulsion. Neutralization with 2.0 g of a 60 % triethanolamine solution gave a final pH value of 8. Finally 1 ,0 g of a 30 % actives solution of parabenes in 2- phenoxyethanol was added as a preservative. The resulting product was a optically clear emulsion with an average particle size of 66 nm, a silicone content of 20 %, a total solids content of 38.9 %. After 5 weeks at 40 °C the emulsion was unchanged. Diluting the emulsion 5:2 with 10 % sodium chloride solution
gave no change. The emulsion was also stable to dilution with a 10 % solution of sodium carbonate.
Comparative Example 2
Emulsion of Siloxane (2) and alkylpolyglycoside with an APG/Siloxane ratio of 0.75 without co-surfactant
30 g of a 50 % actives solution of an alkylpolyglycosides (Plantacare 2000 UP), 20 g of the aminopolysiloxane from Example 2 and 46 g deionized water were mixed at room temperature. The mixture was acidified to a pH value of 5 with
1 .7 g of acetic acid resulting in a clear microemulsion. Neutralization with 2.0 g of a 60 % triethanolamine solution gave a final pH value of 7.5. Finally 1.0 g of a 30 % actives solution of parabenes in 2-phenoxyethanol was added as a preservative. The resulting product was slightly turbid, yellowish emulsion with a silicone content of 20 %, a total solids content of 37.7 %, a viscosity at 23 °C of 16,5 mPa*s. After standing a room temperature for several days a minor oil phase separated to the surface. Diluting the emulsion 5:2 with 10 % sodium chloride solution caused the emulsion to break, separating into two phases.
Example 3
Microemulsion of Siloxane (2), alkylpolyglycoside and a cationic cosurfactant with an APG/Siloxane ratio of 0.83
20 g of a 62 % actives solution of an alkylpolyglycoside (Glucopon 2 5 UP), 15 g of the aminopolysiloxane from Example 2.5 g of a 75 % actives solution of Dico- coalkyldimethylammonium chloride (Arquad 2C-75) and 58.8 g of deionized water were mixed at room temperature, resulting a slightly opaque emulsion. The emulsion was acidified to a pH value of 5 with 1.2 g of acetic acid and heated to 60 °C for 1 hour, becoming a microemulsion. The resulting product was optically clear, with a light yellow color, an average particle size of 87 nm, a silicone content of 15 %, a total solids content of 33.85 % and a viscosity at 23 °C of 8.15
mPa*s. After 5 weeks at 40 °C the emulsion was unchanged. Diluting the emulsion 5:2 with 10% sodium chloride solution gave no change. The emulsion was also stable to neutralization with either triethanolamine or sodium carbonate to a pH value of 8. In each case the resulting neutralized emulsion was clear and stable at 40 °C for in excess of 6 weeks.
Comparative Example 3
Emulsion of Siloxane (2) and alkylpolyglycoside with an APG/Siloxane ratio of 0.83 without co-surfactant
20 g of a 62 % actives solution of an alkylpolygycosides (Glucopon 215 UP),
15 g of the aminopolysiloxane from example 2 and 63 g water were mixed at room temperature. The mixture was acidified to a pH value of 5 with 1.2 g of acetic acid and heated to 70 °C for 60 minutes. The resulting emulsion was optically clear with a pH value of 5, an average particle size of 63 nm, a silicone content of
15.3 %, a total solids content of 28.6 %, a viscosity at 23 °C of 4.4 mPa*s. Diluting the emulsion 5:2 with a 10 % sodium chloride solution caused the emulsion to break, separating into two phases. Neutralization with sodium carbonate to a pH value of 8 caused phase separation. Neutralization of the emulsion to a pH of 8 with triethanolamine and dilution 5:2 with 10 % NaCI resulted in a slightly cloudy emulsion with an average particle size of 122 nm.
Example 4
Microemulsion of Siloxane (2), alkylpolyglycoside and an zwitterionic cosurfactant with an APG/Siloxane ratio of 0.75
25,5 g of a 50 % actives solution of a mixture of alkylpolyglycosides, 6.2 g of a 33 % actives solution of Cocoamidopropyl betaine (Amonyl 380 BA), 17 g of the aminopolysiloxane from example 2 and 50.3 g water were mixed at room temperature, resulting a clear microemulsion. The pH was adjusted with 1 .3 g
acetic acid and 2.0 g of a 60 % triethanolamine solution. The resulting product was a clear, slightly yellow microemulsion with a silicone content of 16.6 %, a total solids content of 33.3 %, a density of 1 .03 g/ml, a viscosity at 25 °C of
6.2 mPa*s, a refractive index nD 2o = 1 .3432 and a pH value of 7.5. After in 12 weeks at 40 °C the emulsion was still transparent with a light yellow color. Diluting the emulsion 1 :9 with deionized water gave a clear emulsion that remained stable both at room temperature and at 40 °C for in excess of 4 weeks. Diluting the emulsion 5:2 with 10 % sodium chloride solution gave no change.
Example 5
Microemulsion of Siloxane (2), alkylpolyglycoside and a zwitterionic cosurfactant with an APG/Siloxane ratio of 0.93
37 g of a 50 % actives solution of a mixture of alkylpolyglycosides, 9 g of a 33 % actives solution of Cocoamidopropyl betaine (Amonyl 380 BA), 20 g of the aminopolysiloxane from example 2 and 32.3 g water were mixed at room temperature, resulting a clear microemulsion. The pH was adjusted with 1 .7 g acetic acid to give a clear, slightly yellow microemulsion with a silicone content of 20 %, a total solids content of 43.3 %, a viscosity at 23 °C of 32 mPa*s, and a pH value of 5. Diluting the emulsion 1 :9 with deionized water gave a clear emulsion that remained stable both at room temperature and at 40 °C for in excess of 4 weeks. Diluting the emulsion 5:2 with 10 % sodium chloride solution gave no change. The emulsion was also stable to neutralization with either triethanolamine or sodium carbonate to a pH value of 8.
Example 6
Microemulsion of Siloxane (2), alkylpolyglycoside and a cationic cosurfactant with an APG/Siloxane ratio of 1.09
37 g of a 50 % actives solution of a mixture of alkylpolyglycosides (available as Glucopon 600 UP from Cognis), 26 g of a 30 % actives solution of a hexadecyl- trimethylammonium chloride (available as Arquad 16-29 from Akzo Nobel), 17 g
of a the aminopolysiloxane from example 2 and 16 g of deionized water were mixed at room temperature. The pH was adjusted with 1.3 g of acetic acid. The resulting microemulsion was clear, yellow, and had a total solids content of 48.6 %, a viscosity at 23 °C of 74 mPa*s and a density at 20 °C of 1.034 g/ml, a refractive index nD 2o = 1.3970 and a pH value of 5. Diluting the emulsion 1 :9 with deionized water gave a clear emulsion that remained stable both at room temperature and at 40 °C for in excess of 4 weeks.
Example 7
Microemulsion of Siloxane (2), alkylpoiyglycoside and a cationic cosurfactant with an APG/Siloxane ratio of 0.55
45 g of a 62 % actives solution of an alkylpoiyglycoside (Glucopon 215 UP), 51 g of the aminopolysiloxane from example 2, 30 g of a 75 % actives solution of dicocoalkyldimethylammonium chloride (Arquad 2C-75), and 1 14 g of deionized water were mixed using a mechanical stirrer. The resulting emulsion was acidified to a pH value of 5 with 3.9 g of acetic acid. Neutralization with 7.5 g of a a 60 % triethanolamine solution gave final pH value of 7.5. Finally 2.5 g of a 30 % actives solution of parabenes in 2-phenoxyethanol was added as a preservative. The resulting product was a clear, slightly yellow microemulsion with a silicone content of 20 %, a total solids content of 42,7 %, a viscosity at 23 °C of 257 mPa*s and a refractive index nD 20 = 1 .3885. After in 12 weeks at 40 °C the emulsion was unchanged. Diluting the emulsion 1 :9 with deionized water gave a clear emulsion that remained stable both at room temperature and at 40 °C for in excess of 4 weeks.
Example 8
Microemulsion of Siloxane (2), alkylpoiyglycoside and an aminoxide cosurfactant with an APG/Siloxane ratio of 1.08
37 g of a 50 % actives solution of an alkylpolyglycosides (Plantacare 2000 UP), 17 g of a the aminopolysiloxane from example 2.9 g of a 30 % actives solution of
a Ci2-Ci4-alkyldimethylamine oxide (available as Genaminox LA from Clariant) and 32 g of deionized water were mixed at room temperature. The mixture was acidified to a pH value of 5 with 1 .3 g of acetic acid and heated to 60 °C for 1 hour. Neutralisation with 2.0 g of a 60 % triethanolamine solution gave a final pH value of 7.5. Finally 0,5 g of a 30 % actives solution of parabenes in 2-phen- oxyethanol was added as a preservative. The resulting product was a optically clear emulsion with a silicone content of 17 %, a total solids content of 43.2 %, a viscosity at 23 °C of 21.4 mPa*s. After in 8 weeks at 40 °C the emulsion was unchanged. Diluting the emulsion 5:2 with 10 % sodium chloride solution gave no change.
Example 9
Microemulsion of Siloxane (2), alkylpolyglycoside and a zwitterionic cosurfactant with an APG/Siloxane ratio of 0.75
24 g of a 62 % actives solution of alkylpolyglycosides (commercially available as Glucopon 215 UP from Cognis Carechemicals), 20 g of a aminopolysiloxane from example 2, 4.5 g of 33 % actives solution of Cocoamidopropyl betaine (available as Dehyton K from Cognis) and 49 g of deionized water were mixed at room temperature. The mixture was acidified to a pH value of 5 with 2.0 g of acetic acid resulting in a clear microemulsion. The resulting product was a glas clear, slightly yellow emulsion with a silicone content of 20 %, a total solids content of 37.3 %. After in 5 weeks at 40 °C the emulsion was unchanged. Diluting the emulsion 5:2 with 10 % sodium chloride solution gave no change. Neutralization with a 10 % solution of sodium carbonate to a pH value of 8 resulted in a clear, slightly yellow microlemulsion that was stable for months.
Example 10
Hair Shampoo
A clear shampoo was formulated with 15.4 % of Plantacare 2000 UP, a 50 % solution of alkylpolyglucosides, 7.8 % of a 33 % actives solution of cocoamido-
propyl betaine, 5.2 % of a sodium Ci2-Ci8-alkylsulfate (available as Sulfopon 1216G from the Cognis), 1 .5 % Xanthan Gum, 5.0 % of the inventive emulsion from Example 2, 0.5 % citric acid and water. The resultant shampoo was applied to standardized hair switches in comparison with the same formulation, but without silicone. Hair treated with the inventive shampoo was soft to the touch and very easy to comb. The hair treated with the silicone-free shampoo was noticeably less soft to the touch and could only be combed with difficulty.
Example 11
Hair Conditioner
A clear hair conditioner was formulated with 1.5 % cetearyl alcohol, 0.3 % cetrimonium chloride, 1.5 % hydroxyethylcellulose, 1 .0 % glyceryl stearate, 5.0 % of the inventive emulsion from Example 2 and water. The resultant hair conditioner was a clear, viscous liquid. The formulation was applied to standardized hair switches that had been washed with a conditioner-free standard shampoo. Hair treated with the inventive conditioner was soft to the touch and very easy to comb. The untreated, washed hair was noticeable less soft to the touch and could only be combed with difficulty.
Comparative Example 4
Emulsion of Siloxane (2), alkylpolyglycoside and 1-pentanol with an APG/Siloxane ratio of 0.35 (according to US 5,466,746, Example 6)
12 g of a 50 % actives solution of a mixture of alkylpolyglycosides (Plantacare 2000 UP), 17 g of a the aminopolysiloxane from Example 2 and 46 g of deionized water were mixed using low shear at room temperature. The mixture was acidified with 1.0 g of acetic acid. The 3.2 g of 1 -pentanol and 30.5 g of deionized water were added with continued agitation. The resulting emulsion was white, opaque and had an average particle size of 384 nm. The emulsion had a silicone content of 17 %, a total solids content of 22.7 %, a refractive index nD2o = 1.3604
and a pH value of 5. Diluting the emulsion 1 :9 with deionized water resulted in the rapid separation at room temperature into two phases.
Comparative Example 5
Emulsion of Siloxane (2), alkylpolyglycoside and a zwitterionic cosurfactant with an APG/Siloxane ratio of 0,45
15.3 g of a 50 % actives solution of a mixture of alkylpolyglycosides (Plantacare 2000 UP), 4.1 g of a 33% actives solution of cocoamidopropyl betaine, 17 g of a the aminopolysiloxane from Example 2 and 61 g of deionized water were mixed a room temperature. The emulsion was acidified to a pH value of 5 with 1 .3 g of acetic acid, resulted in a slightly turbid emulsion. Subsequent neutralization with 2.0 g of a a 60 % triethanolamine solution did not effect the emulsion. Heating the emulsion to 80 °C for 1 hour caused the emulsion to become clear, but upon cooling to room temperature the emulsion became opaque again. The resulting emulsion had an average particle size of 132 nm, was opaque, had a silicone content of 17 %, a total solids content of 30,0 %, a viscosity at 23 °C of 4,6 mPa*s, a refractive index nD 2o = 1.3685 and a pH value of 8. Diluting the emulsion 1 :9 with deionized water gave an opaque macroemulsion.
Comparative Example 6
Macroemulsion of Siloxane (2) with an Zwitterionic Co-Surfactant without APG
56g of a 33 % actives solution of cocoamidopropyl betaine, 17 g of a aminopolysiloxane from Example 2 and 24 g of deionized water were mixed at room temperature. The pH was adjusted to a value of 5 with 1 ,3 g of acetic acid and then neutralized to a pH of 7,5 with 2,0 g of a 60 % solution of triethanolamine. The resulting emulsion had an average particle size of 13,5 microns, and was off- white and opaque.
Comparative Example 7
Emulsion of Siloxane (1 ), alkylpolyglycoside and the anionic co-surfactant alkyl cocoyi isethionate with an APG/Siloxane ratio of 0.83
20 g of a 62 % actives solution of an alkylpolyglycoside (Glucopon 215 UP),
2 g of sodium cocoyi isethionate (Jordapon CI Prill available from BASF), 15 g of the aminopolysiloxane from Example 1 and 61 g of deionized water were mixed a room temperature. The emulsion was acidified to a pH value of 5 with 1 .2 g of acetic acid, resulted in a turbid emulsion. Heating the emulsion to 75 °C for 1 hour resulted in no change. Neutralization with 2.0 g of a a 60% triethanolamine solution gave final pH value of 7.5. Upon standing at room temperture the resulting emulsion had an average particle size of 204 nm, was opaque, had a silicone content of 15 %, and a total solids content of 32.84 %. Diluting the emulsion 1 :9 with deionized water gave an opaque macroemulsion.
Comparative Example 8
Emulsion of Siloxane (1 ), alkylpolyglycoside and glycerine monoloeate with an APG/Siloxane ratio of 0.83
20 g of a 62 % actives solution of an alkylpolyglycoside (Glucopon 215 UP),
2 g of glycerin monooleate (Cithrol GMO from Croda GmbH), 15 g of the aminopolysiloxane from Example 1 and 60.5 g of deionized water were mixed a room temperature. The emulsion was acidified to a pH value of 5 with 1 .2 g of acetic acid, resulted in a slightly turbid emulsion. Diluting the emulsion 5:2 with 10 % sodium chloride solution gave an opaque macroemulsion. Storage at room temperature caused the emulsion to separate into two phases within less than 2 weeks.
Comparative Example 9
Emulsion of Siloxane (2), alkylpolyglycoside and sodium laurylsarcosinate with an APG/Siloxane ratio of 1.09
37 g of a 50 % actives solution of an alkylpolyglycoside (Plantacare 2000 UP),
9 g of a 30 % actives solution of sodium laurylsarcosinate (Crodasinic LS 30),
17 g of the aminopolysiloxane from Example 2 and 33 g of deionized water were mixed a room temperature. The emulsion was acidified to a pH value of 5 with 1 .3 g of acetic acid, resulting in a slightly turbid emulsion. Neutralization with 2.0 g of a a 60 % triethanolamine solution gave final pH value of 7.5. Finally 0.5 g of a 30 % actives solution of parabenes in 2-phenoxyethanol was added as a preservative. A minor oil phase separated to the surface of the emulsion after standing a room temperature for several weeks. Storage at 40 °C for more than 2 weeks caused the emulsion to separate into two phases.
Comparative Example 10
Emulsion of Siloxane (1 ), alkylpolyglycoside and sorbitan monolaurate with an APG/Siloxane ratio of 0.83
Γ) n nf a R9 %
1. _> I _ I P , "i, 9— n of sorbitan monolaurate (Span 20 from Uniqema), 15 g of a the aminopolysiloxane from Example 1 and 61 .2 g of deionized water were mixed a room temperature. The emulsion was acidified to a pH value of 5 with 1.2 g of acetic acid and neutralization with 2.0 g of a a 60 % triethanolamine solution gave final pH value of 7.5. The resulting emulsion was slightly turbid. Diluting the emulsion 5:2 with 10 % sodium chloride solution gave an emulsion with an average particle size of 204 nm. Neutralization with with sodium carbonate to a pH value of 8 gave an opaque macroemulsion.
Comparative Example 11
Emulsion of Siloxane (2), alkylpolyglycoside and an alcohol ethoxylate with an APG/Siloxane ratio of 1.09
37 g of a 50 % actives solution of a mixture of alkylpolyglycosides (Plantacare 2000 UP), 3 g of a secondary alcohol ethoxylate with a HLB value of 12,4 (available as Tergitol 15-S-7 from Dow Chemical) , 17 g of a the aminopoly-
siloxane from Example 2 and 37 g water were mixed at room temperature, resulting a clear microemulsion. The pH was adjusted using acetic acid. Finally 1 .0 g of a 30 % actives solution of parabenes in 2-phenoxyethanol was added as a preservative. The resulting product was a clear, slightly yellow microemulsion with a silicone content of 17 %, a total solids content of 40.5 %, a density of 1 .04 g/ml, a viscosity at 23 °C of 19,7 mPa*s, a refractive index nD 20 = 1 .3827 and a pH value of 5.5. The resulting microemulsion was completely clear, but slightly yellow. Neutralization with sodium carbonate to a pH value of 8 caused phase separation. Diluting the emulsion 1 :9 with deionized water gave a clear emulsion that remained stable both at room temperature and at 40 °C for in excess of 4 weeks. Diluting the emulsion 5:2 with 10% sodium chloride solution gave no change.
Comparative Example 12
Emulsion of Siloxane (1), alkylpolyglycoside and an alcohol ethoxylate with an APG/Siloxane ratio of 0.83
20 g of a 62 % actives solution of an alkylpolyglycoside (Glucopon 215 UP),
3 g of lauryl alcohol ethoxylate with an HLB value of 16.7 (Volpo L23 from Croda), 15 g of a the aminopolysiloxane from Example 1 and 58.3 g of deionized water were mixed a room temperature. The emulsion was acidified to a pH value of 5 with 1 .2 g of acetic acid and neutralization with 2.0 g of a a 60 % triethanolamine solution gave final pH value of 7. The resulting emulsion was clear, but slightly yellow. Diluting the emulsion 5:2 with 10 % sodium chloride solution caused the emulsion to become slightly turbid. Diluting the emulsion 10: 1 with 10 % sodium carbonate solution caused the emulsion to break, separating into two phases.
Claims
1. Aqueous silicone emulsions, comprising:
a) 100 parts by weight of one or more polyorganosiloxanes which contain one or more polar groups on Si-C-bonded hydrocarbon radicals,
b) > 50 - 150 parts by weight of one or more alkylpolyglucosides, c) 0.1 - 150 parts by weight of one or more co-surfactants selected from the group consisting of amphoteric or zwitterionic surfactants, amine oxide surfactants and cationic surfactants,
d) water.
2. Aqueous emulsions according to claim 1 , in which the polar groups bonded to Si-C-bonded hydrocarbon radicals are selected from the group consisting of ammo, ammonium, epoxy, hydroxyi, amido, mercapto, carboxyl, carboxylic acid ester, sulphonic acid, and sulphonic acid ester groups or salts thereof.
3. Aqueous emulsions according to any of the previous claims, in which the polar groups bonded to Si-C-bonded hydrocarbon radicals are selected from the group consisting of amino and ammonium groups.
4. Aqueous emulsions according to claim 1 , in which the polyorganosiloxanes are selected from the group of: a1 ) polyorganosiloxanes, wherein the polar groups are part of the backbone chain, between terminal groups of the polyorganosiloxane moieties in the backbone chain, and a2) polyorganosiloxanes, wherein the polar groups are pendant groups attached to the polyorganosiloxane moieties in the backbone chain,
5. Aqueous emulsions according to claim 4, in which the polyorganosiloxanes a) are selected from the group polyamino- and/or polyammonium-polysiloxane compounds a1 ) and/or amino- and/or ammonium-polysiloxane compounds a2).
Aqueous emulsions according to claim 4, in which the polyorganosiloxanes a2) contain at least 0,3 mmol/g of polar groups.
Aqueous emulsions according to any of the previous claims, in which the co-surfactant is selected from amphoteric or zwitterionic surfactants and cationic surfactants.
8. Aqueous emulsions according to any of the previous claims, in which the co-surfactant is free of oxyalkylene groups.
9. Aqueous emulsions according to any of the previous claims, in which the weight ratio of the cosurfactants to the alkylpolyglucosides is between 0,001 and 0,5.
10. Aqueous emulsions according to any of the previous claims, in which the weight ratio of the alkylpolyglucosides to the polyorganosiloxanes is less than 1.
1 1 , Aqueous emulsions according to any of the previous claims, comprising > 50 - 120 parts by weight of one or more alkylpolyglucosides per 100 parts by weight of the polyorganosiloxane(s).
12. Aqueous emulsions according to any of the previous claims, wherein the alkylpolyglucosides b) are selected form at least one compound of the following formula (II)
AO-[G]p (II) wherein the unit 'G' is a sugar unit, 'p' is an average number of 1 to 10, and A is selected from the group of straight- or branched-chain C4 - C24- alkyl or alkenyl groups.
13. Aqueous emulsions according to any of the previous claims, comprising 0.1 - 50 parts of one or more co-surfactants.
Aqueous emulsions according to any of the previous claims, having a volume average d50 droplet size of less than 120 nm, measured by laser scattering method.
15. Use of the aqueous emulsions according to any of the previous claims for the manufacture of cosmetic compositions, as carrier for chemicals, for the treatment of surfaces or substrates, which include fibers or fibrous materials, carbon or glass fibers or substrates, painted surfaces or glass or plastic surfaces, construction materials, for the manufacture of cleansing or polishing compositions.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09172259.5 | 2009-10-05 | ||
| EP09172259 | 2009-10-05 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2011042409A2 true WO2011042409A2 (en) | 2011-04-14 |
| WO2011042409A3 WO2011042409A3 (en) | 2011-09-22 |
Family
ID=42087827
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2010/064786 WO2011042409A2 (en) | 2009-10-05 | 2010-10-05 | Aqueous emulsions of polyorganosiloxanes |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011042409A2 (en) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013000592A1 (en) * | 2011-06-30 | 2013-01-03 | Evonik Goldschmidt Gmbh | Microemulsion of quaternary polysiloxanes containing ammonium groups, production and use thereof |
| EP2557181A1 (en) | 2011-08-12 | 2013-02-13 | LANXESS Deutschland GmbH | Method for hydrophobic finishing of substrates containing collagen fibre |
| WO2013082112A1 (en) * | 2011-11-29 | 2013-06-06 | Dow Corning Corporation | Aminofunctional silicone emulsions |
| WO2012130413A3 (en) * | 2011-04-01 | 2014-04-17 | Cognis Ip Management Gmbh | Hair care agent |
| CN103946444A (en) * | 2011-11-29 | 2014-07-23 | 道康宁公司 | Aminofunctional silicone emulsions for fiber treatments |
| WO2015013248A1 (en) * | 2013-07-26 | 2015-01-29 | The Procter & Gamble Company | Amino silicone nanoemulsion |
| US9717676B2 (en) | 2013-07-26 | 2017-08-01 | The Procter & Gamble Company | Amino silicone nanoemulsion |
| EP3501488A1 (en) | 2017-12-21 | 2019-06-26 | Momentive Performance Materials GmbH | Aqueous silicone polymer compositions |
Citations (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2438091A (en) | 1943-09-06 | 1948-03-16 | American Cyanamid Co | Aspartic acid esters and their preparation |
| US2528378A (en) | 1947-09-20 | 1950-10-31 | John J Mccabe Jr | Metal salts of substituted quaternary hydroxy cycloimidinic acid metal alcoholates and process for preparation of same |
| US2658072A (en) | 1951-05-17 | 1953-11-03 | Monsanto Chemicals | Process of preparing amine sulfonates and products obtained thereof |
| DE1943689A1 (en) | 1968-09-03 | 1970-03-12 | Rohm & Haas | Alkyl oligosaccharides and their mixtures with alkyl glucosides and alkanols |
| US3929678A (en) | 1974-08-01 | 1975-12-30 | Procter & Gamble | Detergent composition having enhanced particulate soil removal performance |
| US4146499A (en) | 1976-09-18 | 1979-03-27 | Rosano Henri L | Method for preparing microemulsions |
| US4529586A (en) | 1980-07-11 | 1985-07-16 | Clairol Incorporated | Hair conditioning composition and process |
| US4559227A (en) | 1984-10-31 | 1985-12-17 | Dow Corning Corporation | Conditioning shampoo containing amine functional polydiorganosiloxane |
| US4563347A (en) | 1982-05-20 | 1986-01-07 | Dow Corning Corporation | Compositions used to condition hair |
| US4586518A (en) | 1984-08-06 | 1986-05-06 | Dow Corning Corporation | Hair setting method using aminoalkyl substituted polydiorganosiloxane |
| US4620878A (en) | 1983-10-17 | 1986-11-04 | Dow Corning Corporation | Method of preparing polyorganosiloxane emulsions having small particle size |
| EP0282720A2 (en) | 1987-02-18 | 1988-09-21 | Th. Goldschmidt AG | Polyquaternary polysiloxanes, their preparation and their use in cosmetic compounds |
| EP0418479A1 (en) | 1989-08-04 | 1991-03-27 | Hüls Aktiengesellschaft | Emulsifier for shelf stable aqueous organosiloxane and/or paraffine oil emulsions |
| US5035832A (en) | 1989-05-17 | 1991-07-30 | Kao Corporation | Mild liquid aqueous detergent compositions containing an alkylsaccharide surface active agent and a silicone derivative |
| US5057572A (en) | 1987-04-24 | 1991-10-15 | Ciba-Geigy Corporation | Aqueous, finely divided to optically clear, thermally and mechanically stable silicone emulsions, a process for their preparation and their use |
| US5104646A (en) | 1989-08-07 | 1992-04-14 | The Procter & Gamble Company | Vehicle systems for use in cosmetic compositions |
| WO1992006154A1 (en) | 1990-09-28 | 1992-04-16 | The Procter & Gamble Company | Polyhydroxy fatty acid amide surfactants to enhance enzyme performance |
| US5106609A (en) | 1990-05-01 | 1992-04-21 | The Procter & Gamble Company | Vehicle systems for use in cosmetic compositions |
| DE4131551A1 (en) | 1991-09-21 | 1993-03-25 | Pfersee Chem Fab | Aq. dispersions of amino- or amido-polysiloxane(s) - contain alkyl-poly:glycoside(s) as dispersants, and are useful for treatment of fibre materials, esp. textiles |
| US5244598A (en) | 1991-09-13 | 1993-09-14 | General Electric Company | Method of preparing amine functional silicone microemulsions |
| US5258451A (en) | 1990-06-07 | 1993-11-02 | Shin-Etsu Chemical Co., Ltd. | Method for the preparation of an aqueous emulsion of organopolysiloxane |
| US5268126A (en) | 1989-08-04 | 1993-12-07 | Huels Aktiengesellschaft | Emulsifiers for the preparation of aqueous polysiloxane emulsions and aqueous polysiloxane-paraffin oil emulsions with long shelf lives |
| DE4229922A1 (en) | 1992-09-08 | 1994-03-10 | Kao Corp Gmbh | Hair conditioning compsn., giving good hair vol. and soft shine - contains quat. ammonium cpd., alkyl:poly:glucoside non-volatile silicone and opt. amino-functional polysiloxane |
| US5302657A (en) | 1990-02-16 | 1994-04-12 | Wacker-Chemie Gmbh | Highly dispersed organopolysiloxane emulsions |
| DE4318536A1 (en) | 1993-06-04 | 1994-12-08 | Bayer Ag | Siloxanyl modified polyhydroxylated hydrocarbons |
| DE4318537A1 (en) | 1993-06-04 | 1994-12-08 | Bayer Ag | Cationic siloxanyl modified polyhydroxylated hydrocarbons |
| US5409628A (en) | 1991-08-22 | 1995-04-25 | Goldwell, Ag | Hair shampoo |
| US5466746A (en) | 1993-03-04 | 1995-11-14 | Wacker-Chemie Gmbh | Emulsions of organopolysiloxanes containing polar groups with alkyl polyglycosides as emulsifiers |
| US5683625A (en) | 1994-05-27 | 1997-11-04 | General Electric Company | Method of preparing microemulsions |
| US5690920A (en) | 1990-11-15 | 1997-11-25 | L'oreal | Foamable washing composition based on selected insoluble silicones and an alkylpolyglycoside, and cosmetic and dermatological uses thereof |
| WO1998008436A1 (en) | 1996-08-30 | 1998-03-05 | Plasto S.A. | Removable hygienic mouthpiece |
| US5981681A (en) | 1996-03-04 | 1999-11-09 | Witco Corporation | Silicone aminopolyalkyleneoxide block copolymers |
| US6147038A (en) | 1991-05-24 | 2000-11-14 | Dow Corning Corporation | Optically clear hair conditioning compositions containing aminofunctional silicone microemulsions |
| US6240929B1 (en) | 1998-04-01 | 2001-06-05 | L'oreal S.A. | Heterocyclic quaternary polyammonium silicon polymers and their use in cosmetic compositions |
| WO2002010259A1 (en) | 2000-07-27 | 2002-02-07 | Ge Bayer Silicones Gmbh & Co. Kg | Polysiloxane polymers, method for their production and the use thereof |
| WO2002010257A1 (en) | 2000-07-27 | 2002-02-07 | Ge Bayer Silicones Gmbh & Co. Kg | Polyammonium-polysiloxane compounds, methods for the production and use thereof |
| WO2002010256A1 (en) | 2000-07-27 | 2002-02-07 | Ge Bayer Silicones Gmbh & Co. Kg | Mono- or poly-quaternary polysiloxanes |
| DE10036533A1 (en) | 2000-07-27 | 2002-02-14 | Ge Bayer Silicones Gmbh & Co | Production of polyquaternary polysiloxanes, useful as wash-resistant fabric conditioners, comprises reacting hydrogen-terminal dimethylpolysiloxane with olefin-terminal epoxide, and reacting with mixture of tertiary and ditertiary amines |
| DE10036532A1 (en) | 2000-07-27 | 2002-02-21 | Ge Bayer Silicones Gmbh & Co | Novel approximatelya,approximatelyc-aminofunctionalized polysiloxanes are useful in cosmetic formulations for skin and hair care, in polishes and as softeners. |
| DE10036522A1 (en) | 2000-07-27 | 2002-02-21 | Ge Bayer Silicones Gmbh & Co | Novel linear aminoacid modified polyquaternary polysiloxanes are useful in cosmetic formulations for skin and hair care, in polishes and as softeners |
| US6451905B2 (en) | 1999-02-16 | 2002-09-17 | Crompton Corporation | Shear stable aminosilicone emulsions |
| US6547490B2 (en) | 2000-07-18 | 2003-04-15 | Solvay Interox Gmbh | Coated metal peroxides |
| EP1972817A2 (en) | 2007-03-22 | 2008-09-24 | LuK Lamellen und Kupplungsbau Beteiligungs KG | Disc spring lever component for actuating friction clutch with integrated wear compensation and self-adjusting friction clutch |
-
2010
- 2010-10-05 WO PCT/EP2010/064786 patent/WO2011042409A2/en active Application Filing
Patent Citations (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2438091A (en) | 1943-09-06 | 1948-03-16 | American Cyanamid Co | Aspartic acid esters and their preparation |
| US2528378A (en) | 1947-09-20 | 1950-10-31 | John J Mccabe Jr | Metal salts of substituted quaternary hydroxy cycloimidinic acid metal alcoholates and process for preparation of same |
| US2658072A (en) | 1951-05-17 | 1953-11-03 | Monsanto Chemicals | Process of preparing amine sulfonates and products obtained thereof |
| DE1943689A1 (en) | 1968-09-03 | 1970-03-12 | Rohm & Haas | Alkyl oligosaccharides and their mixtures with alkyl glucosides and alkanols |
| US3929678A (en) | 1974-08-01 | 1975-12-30 | Procter & Gamble | Detergent composition having enhanced particulate soil removal performance |
| US4146499A (en) | 1976-09-18 | 1979-03-27 | Rosano Henri L | Method for preparing microemulsions |
| US4529586A (en) | 1980-07-11 | 1985-07-16 | Clairol Incorporated | Hair conditioning composition and process |
| US4563347A (en) | 1982-05-20 | 1986-01-07 | Dow Corning Corporation | Compositions used to condition hair |
| US4620878A (en) | 1983-10-17 | 1986-11-04 | Dow Corning Corporation | Method of preparing polyorganosiloxane emulsions having small particle size |
| US4586518A (en) | 1984-08-06 | 1986-05-06 | Dow Corning Corporation | Hair setting method using aminoalkyl substituted polydiorganosiloxane |
| US4559227A (en) | 1984-10-31 | 1985-12-17 | Dow Corning Corporation | Conditioning shampoo containing amine functional polydiorganosiloxane |
| EP0282720A2 (en) | 1987-02-18 | 1988-09-21 | Th. Goldschmidt AG | Polyquaternary polysiloxanes, their preparation and their use in cosmetic compounds |
| US5057572A (en) | 1987-04-24 | 1991-10-15 | Ciba-Geigy Corporation | Aqueous, finely divided to optically clear, thermally and mechanically stable silicone emulsions, a process for their preparation and their use |
| US5035832A (en) | 1989-05-17 | 1991-07-30 | Kao Corporation | Mild liquid aqueous detergent compositions containing an alkylsaccharide surface active agent and a silicone derivative |
| EP0418479A1 (en) | 1989-08-04 | 1991-03-27 | Hüls Aktiengesellschaft | Emulsifier for shelf stable aqueous organosiloxane and/or paraffine oil emulsions |
| US5268126A (en) | 1989-08-04 | 1993-12-07 | Huels Aktiengesellschaft | Emulsifiers for the preparation of aqueous polysiloxane emulsions and aqueous polysiloxane-paraffin oil emulsions with long shelf lives |
| US5104646A (en) | 1989-08-07 | 1992-04-14 | The Procter & Gamble Company | Vehicle systems for use in cosmetic compositions |
| US5302657A (en) | 1990-02-16 | 1994-04-12 | Wacker-Chemie Gmbh | Highly dispersed organopolysiloxane emulsions |
| US5106609A (en) | 1990-05-01 | 1992-04-21 | The Procter & Gamble Company | Vehicle systems for use in cosmetic compositions |
| US5258451A (en) | 1990-06-07 | 1993-11-02 | Shin-Etsu Chemical Co., Ltd. | Method for the preparation of an aqueous emulsion of organopolysiloxane |
| WO1992006154A1 (en) | 1990-09-28 | 1992-04-16 | The Procter & Gamble Company | Polyhydroxy fatty acid amide surfactants to enhance enzyme performance |
| US5690920A (en) | 1990-11-15 | 1997-11-25 | L'oreal | Foamable washing composition based on selected insoluble silicones and an alkylpolyglycoside, and cosmetic and dermatological uses thereof |
| US6153569A (en) | 1991-05-24 | 2000-11-28 | Dow Corning Corporation | Optically clear shampoo compositions containing aminofunctional silicone microemulsions |
| US6147038A (en) | 1991-05-24 | 2000-11-14 | Dow Corning Corporation | Optically clear hair conditioning compositions containing aminofunctional silicone microemulsions |
| US5409628A (en) | 1991-08-22 | 1995-04-25 | Goldwell, Ag | Hair shampoo |
| US5244598A (en) | 1991-09-13 | 1993-09-14 | General Electric Company | Method of preparing amine functional silicone microemulsions |
| DE4131551A1 (en) | 1991-09-21 | 1993-03-25 | Pfersee Chem Fab | Aq. dispersions of amino- or amido-polysiloxane(s) - contain alkyl-poly:glycoside(s) as dispersants, and are useful for treatment of fibre materials, esp. textiles |
| DE4229922A1 (en) | 1992-09-08 | 1994-03-10 | Kao Corp Gmbh | Hair conditioning compsn., giving good hair vol. and soft shine - contains quat. ammonium cpd., alkyl:poly:glucoside non-volatile silicone and opt. amino-functional polysiloxane |
| US5466746A (en) | 1993-03-04 | 1995-11-14 | Wacker-Chemie Gmbh | Emulsions of organopolysiloxanes containing polar groups with alkyl polyglycosides as emulsifiers |
| DE4318536A1 (en) | 1993-06-04 | 1994-12-08 | Bayer Ag | Siloxanyl modified polyhydroxylated hydrocarbons |
| DE4318537A1 (en) | 1993-06-04 | 1994-12-08 | Bayer Ag | Cationic siloxanyl modified polyhydroxylated hydrocarbons |
| US5683625A (en) | 1994-05-27 | 1997-11-04 | General Electric Company | Method of preparing microemulsions |
| US5981681A (en) | 1996-03-04 | 1999-11-09 | Witco Corporation | Silicone aminopolyalkyleneoxide block copolymers |
| WO1998008436A1 (en) | 1996-08-30 | 1998-03-05 | Plasto S.A. | Removable hygienic mouthpiece |
| US6240929B1 (en) | 1998-04-01 | 2001-06-05 | L'oreal S.A. | Heterocyclic quaternary polyammonium silicon polymers and their use in cosmetic compositions |
| US6451905B2 (en) | 1999-02-16 | 2002-09-17 | Crompton Corporation | Shear stable aminosilicone emulsions |
| US6547490B2 (en) | 2000-07-18 | 2003-04-15 | Solvay Interox Gmbh | Coated metal peroxides |
| WO2002010259A1 (en) | 2000-07-27 | 2002-02-07 | Ge Bayer Silicones Gmbh & Co. Kg | Polysiloxane polymers, method for their production and the use thereof |
| WO2002010257A1 (en) | 2000-07-27 | 2002-02-07 | Ge Bayer Silicones Gmbh & Co. Kg | Polyammonium-polysiloxane compounds, methods for the production and use thereof |
| WO2002010256A1 (en) | 2000-07-27 | 2002-02-07 | Ge Bayer Silicones Gmbh & Co. Kg | Mono- or poly-quaternary polysiloxanes |
| DE10036533A1 (en) | 2000-07-27 | 2002-02-14 | Ge Bayer Silicones Gmbh & Co | Production of polyquaternary polysiloxanes, useful as wash-resistant fabric conditioners, comprises reacting hydrogen-terminal dimethylpolysiloxane with olefin-terminal epoxide, and reacting with mixture of tertiary and ditertiary amines |
| DE10036532A1 (en) | 2000-07-27 | 2002-02-21 | Ge Bayer Silicones Gmbh & Co | Novel approximatelya,approximatelyc-aminofunctionalized polysiloxanes are useful in cosmetic formulations for skin and hair care, in polishes and as softeners. |
| DE10036522A1 (en) | 2000-07-27 | 2002-02-21 | Ge Bayer Silicones Gmbh & Co | Novel linear aminoacid modified polyquaternary polysiloxanes are useful in cosmetic formulations for skin and hair care, in polishes and as softeners |
| EP1972817A2 (en) | 2007-03-22 | 2008-09-24 | LuK Lamellen und Kupplungsbau Beteiligungs KG | Disc spring lever component for actuating friction clutch with integrated wear compensation and self-adjusting friction clutch |
Non-Patent Citations (2)
| Title |
|---|
| I. ZIMMERMANN, INGENIEUR TECHNIK, vol. 68, no. 4, 1996 |
| MCCUTCHEON'S: "Emulsifiers and Detergents", 1989, M. C. PUBLISHING CO. |
Cited By (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012130413A3 (en) * | 2011-04-01 | 2014-04-17 | Cognis Ip Management Gmbh | Hair care agent |
| WO2013000592A1 (en) * | 2011-06-30 | 2013-01-03 | Evonik Goldschmidt Gmbh | Microemulsion of quaternary polysiloxanes containing ammonium groups, production and use thereof |
| US9138385B2 (en) | 2011-06-30 | 2015-09-22 | Evonik Degussa Gmbh | Microemulsion of polysiloxanes containing quaternary ammonium groups, production and use thereof |
| CN103648629A (en) * | 2011-06-30 | 2014-03-19 | 赢创德固赛有限公司 | Microemulsion of quaternary polysiloxanes containing ammonium groups, production and use thereof |
| KR20140056235A (en) * | 2011-06-30 | 2014-05-09 | 에보니크 데구사 게엠베하 | Microemulsion of quaternary polysiloxanes containing ammonium groups, production and use thereof |
| EP2557181A1 (en) | 2011-08-12 | 2013-02-13 | LANXESS Deutschland GmbH | Method for hydrophobic finishing of substrates containing collagen fibre |
| WO2013023980A1 (en) | 2011-08-12 | 2013-02-21 | Lanxess Deutschland Gmbh | Method for hydrophobing substrates containing collagen fibers |
| CN103946446A (en) * | 2011-11-29 | 2014-07-23 | 道康宁公司 | Aminofunctional silicone emulsions |
| CN103946444A (en) * | 2011-11-29 | 2014-07-23 | 道康宁公司 | Aminofunctional silicone emulsions for fiber treatments |
| JP2015500896A (en) * | 2011-11-29 | 2015-01-08 | ダウ コーニング コーポレーションDow Corning Corporation | Amino-functional silicone emulsion |
| WO2013082112A1 (en) * | 2011-11-29 | 2013-06-06 | Dow Corning Corporation | Aminofunctional silicone emulsions |
| US9849309B2 (en) | 2011-11-29 | 2017-12-26 | Dow Corning Corporation | Aminofunctional organosiloxanes |
| US10143862B2 (en) | 2011-11-29 | 2018-12-04 | Dow Silicones Corporation | Aminofunctional Silicone Emulsions |
| US10245451B2 (en) | 2011-11-29 | 2019-04-02 | Dow Silicones Corporation | Aminofunctional organosiloxanes |
| WO2015013248A1 (en) * | 2013-07-26 | 2015-01-29 | The Procter & Gamble Company | Amino silicone nanoemulsion |
| CN105377227A (en) * | 2013-07-26 | 2016-03-02 | 宝洁公司 | Amino silicone nanoemulsion |
| JP2016534178A (en) * | 2013-07-26 | 2016-11-04 | ザ プロクター アンド ギャンブル カンパニー | Aminosilicone nanoemulsion |
| US9717676B2 (en) | 2013-07-26 | 2017-08-01 | The Procter & Gamble Company | Amino silicone nanoemulsion |
| EP3501488A1 (en) | 2017-12-21 | 2019-06-26 | Momentive Performance Materials GmbH | Aqueous silicone polymer compositions |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2011042409A3 (en) | 2011-09-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2011042409A2 (en) | Aqueous emulsions of polyorganosiloxanes | |
| EP2273971B1 (en) | Oil-in-water organopolysiloxane emulsion composition, cosmetic ingredient comprising this composition, and method of producing a hair cosmetic using this composition | |
| EP1309648B1 (en) | Mono- or poly-quaternary polysiloxanes | |
| CN101970053B (en) | Conditioning shampoo composition | |
| EP1887024B1 (en) | New polysiloxanes with quaternary ammonium groups, method for their manufacture and their use in cleaning and treating formulations | |
| JP4108710B2 (en) | Aqueous pearlescent concentrate | |
| JP6095142B2 (en) | Method for producing shampoo composition | |
| TW201021842A (en) | Composition | |
| DE102005057857A1 (en) | Block-type polyether-modified polysiloxanes and their use for the preparation of cosmetic formulations | |
| CA2068124A1 (en) | Optically clear hair care compositions containing amino-functional silicone microemulsions | |
| EP2729112A2 (en) | Use of microemulsions in cosmetic cleaning compositions | |
| EP2831178A1 (en) | LOW VISCOSITY POLYORGANOSILOXANES COMPRISING QUATERNARY AMMONIUM GROUPS, METHODS FOR THE PRODUCTION AND THE USE THEREOF (ll) | |
| EP2736483A2 (en) | Cosmetic compositions | |
| JPS6316418B2 (en) | ||
| US20090233825A1 (en) | Conditioning shampoo compositions | |
| EP2410978A2 (en) | Aqueous hair and skin cleansing compositions | |
| US20180072817A1 (en) | Topical preparations comprising polyalkoxylated polyols polyester | |
| TW201021838A (en) | Composition | |
| WO2010052070A2 (en) | Composition | |
| KR20140019427A (en) | Hair care agent | |
| WO2010052071A2 (en) | Composition | |
| JP2007535605A (en) | Pyrrolidone carboxyl-modified polysiloxanes that can form water-in-oil emulsions with water-soluble and detergent-soluble substituents | |
| WO2002036081A2 (en) | Compositions comprising hydrophobic silicone oils and alkyl ether carboxylates | |
| EP3448357B1 (en) | Aqueous emulsion based on carbamato-modified organopolysiloxanes | |
| JP2020164467A (en) | Liquid thickener composition comprising polyalkoxylated polyol polyester having guerbet acid moieties |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10760698 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WA | Withdrawal of international application | ||
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10760698 Country of ref document: EP Kind code of ref document: A2 |