WO2014005720A1 - Use of (r)-phenylpiracetam for the treatment of disease-associated fatigue - Google Patents
Use of (r)-phenylpiracetam for the treatment of disease-associated fatigue Download PDFInfo
- Publication number
- WO2014005720A1 WO2014005720A1 PCT/EP2013/001990 EP2013001990W WO2014005720A1 WO 2014005720 A1 WO2014005720 A1 WO 2014005720A1 EP 2013001990 W EP2013001990 W EP 2013001990W WO 2014005720 A1 WO2014005720 A1 WO 2014005720A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- fatigue
- phenylpiracetam
- disease
- day
- parkinson
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/4015—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil having oxo groups directly attached to the heterocyclic ring, e.g. piracetam, ethosuximide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the present invention relates to the efficient treatment of an individual afflicted with fatigue, particularly mental fatigue, associated with certain diseases, particularly diseases of the central nervous system, including Parkinson's disease (PD), the instant treatment comprising administering to the individual an effective amount of (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof.
- PD Parkinson's disease
- This invention relates to a method of treating patients afflicted with certain forms of fatigue associated with diseases, particularly of the central nervous system (CNS), including Parkinson's disease.
- CNS central nervous system
- Fatigue can be experienced both as physical fatigue, affecting the muscles and/or the ability to move, and as mental fatigue, affecting the level of attention, motivation and/or consciousness.
- hepatitis C fatigue in cirrhosis, e.g. primary biliary cirrhosis, fatigue in cachexia (age and tumor related), fatigue in amyotrophic lateral sclerosis (ALS), post-polio fatigue, and fatigue in myasthenia gravis.
- Affected patients experience fatigue either constantly without any relevant physical or mental activity or already after a short or slight effort. That kind of pathological fatigue has significant impact on patients' quality of life and calls for therapy.
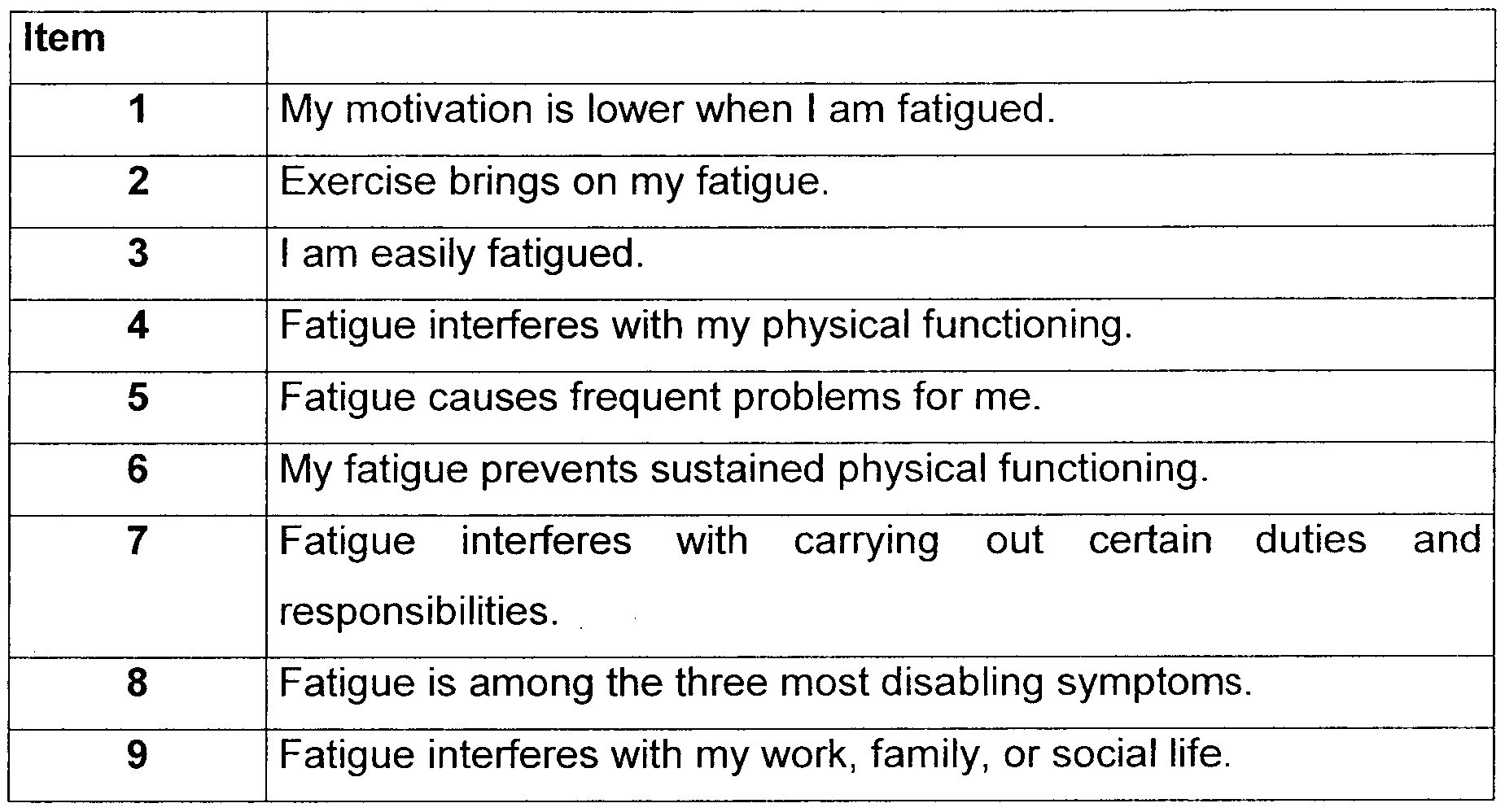
- a well-accepted measurement of this type of fatigue is the Fatigue Severity Scale (FSS), a self-administered unidimensional generic 9-item fatigue rating scale (Krupp et al., Arch Neurol. 46, 1 121-23, 1989). Each item has to be rated on a seven-grade Likert scale with a range from 1 (completely disagree) to 7 (completely agree), see Table 1 .
- the total FSS score is mean score of the scores on the respective 9 items.
- a total FSS score of 4 or higher present over 2 weeks prior to scoring is accepted as definition of presence of disease-orientated fatigue, especially in chronic diseases, e.g. Parkinson's disease.
- Table 1 Fatigue Severity Scale
- Cancer-related fatigue is defined as a persistent, subjective sense of (i) physical, and/or (ii) emotional and/or cognitive (mental) tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and that significantly interferes with usual functioning (National Comprehensive Cancer Network (NCCN) guidelines). Cancer-related fatigue differs from the fatigue that accompanies everyday life, which is usually temporary and relieved by rest.
- NCCN National Comprehensive Cancer Network
- Parkinson's disease is one of the most common chronic neurological diseases.
- the classical symptoms of PD are rigor (muscular stiffness), tremor and bradykinesia (slowness of movements).
- These movement disturbances can nowadays be treated rather effectively with various available PD drugs, particularly in early stages of the disease.
- non-motor symptoms, for which satisfactory treatments are mainly lacking (Gallagher et al., Mov Disord. 2010 Nov 15;25(15):2493-500).
- non-motor symptoms are autonomic dysfunctions (cardiovascular, urinary and gastrointestinal), sleep problems, psychosis, pain, cognitive deficits and fatigue.
- Fatigue in PD is multidimensional including physical, mental and general aspects (Havlikova et al., Parkinsonism Relat Disord. 14, 187-92, 2008; Havlikova et al., Eur J Neurol. ;15(5):475-80, 2008; Havlikova et al., J Neurol Sci. 270, 107-13, 2008).
- the physical dimensions of fatigue in PD are connected to problems regarding mobility and activity of daily living.
- Mental fatigue dimensions affect cognition, emotional well-being, and communication.
- general fatigue is related to bodily discomfort of the patients.
- Karabanov et al. describe an open-label study of the effects of phenylpiracetam on patients suffering from PD (Karabanov et al., Atmosfera. Nervnye Bolezni 4, 29-32, 2009). In the study report, it is mentioned that fatigue was one of the parameters being monitored in the study, and a figure is presented showing a positive effect of phenylpiracetam on the combined parameter "general activity, physical and mental fatigue, asthenization symptoms”. Karabanov neither presents data on the effect of phenylpiracetam on fatigue alone, nor differentiates between disease-related fatigue and "physiological" fatigue.
- Kalinskij and Nazarov describe the effect of phenylpiracetam on fatigability in the treatment of asthenic syndrome (Kalinskij and Nazarov, Zh Nevrol Psikhiatr Im SS Korsakova, 107, 61-63, 2007), and Akhapkina et al. provide additional data from a clinical study Efficacy of Phenotropil in the treatment of asthenic syndrome and chronic fatigue syndrome (Akhapkina et al., Atmosfera. Nervnye Bolezni 3, 28-31 , 2004). Karabanov et al.
- the so-called “Parkinson Fatigue” is an outstanding embodiment of disease-associated fatigue seen exclusively in PD patients.
- Using common rating scales for fatigue causes problems in PD patients, as the critical assessment of Friedman JH et al (Mov. Disorders, 25, 805-822, 2010) revealed.
- the "Parkinson Fatigue Scale” developed by Brown RG et al. (Parkinsonism Related Disorders 2005, 1 1 (1):49-55) excludes emotional and cognitive features that may occur as part of the fatigue experience but which may also occur independently in Parkinsonism.
- the 16 specific items of this scale are reported by the affected patients themselves and allow a clear separation of the Parkinson-specific type of fatigue from other types of fatigue, wherein the cut-off point is at 7 (binary calculation).
- the Parkinson Fatigue Score is not ideally suited for analyzing mental fatigue associated with Parkinson's disease alone.
- the present invention relates to a method of treating disease-associated fatigue, particularly disease-associated mental fatigue, in a subject in need thereof, comprising the step of administering an effective amount of (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof.
- (R)- phenylpiracetam is present as (R)-phenylpiracetam hydrochloride.
- the disease-associated fatigue is fatigue in Parkinson's disease.
- the disease-associated fatigue is mental fatigue in Parkinson's disease.
- the disease-associated fatigue is fatigue in cancer.
- the disease-associated fatigue is mental fatigue in cancer.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 1 mg to about 400 mg/day.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 25 mg to about 350 mg/day.
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 50 mg to about 300 mg/day.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered once a day, particularly at a dose of about 200 mg once a day.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a multiple dose, for example twice a day (b.i.d.), or three times a day, particularly twice a day, particularly at a dose of about 100 mg twice a day.
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from starting from 50 mg and increasing the dose in 50 mg steps until the desired therapeutic efficacy is reached, but maximally to 400 mg/day.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in an oral formulation.
- the present invention relates to a pharmaceutical composition
- a pharmaceutical composition comprising (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof for use in the treatment of disease-associated fatigue.
- (R)-phenylpiracetam is present as (R)-phenylpiracetam hydrochloride.
- the treatment comprises administering (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof in a range from about 1 mg to about 400 mg/day.
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in a range from about 25 mg to about 350 mg/day.
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in a range from about 50 mg to about 300 mg/day, particularly in a range from about 50 mg to about 150 mg/day.
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof e.g. , (R)- phenylpiracetam hydrochloride
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from starting from about 50 mg and increasing the dose in 50 mg steps until the desired therapeutic efficacy is reached, but maximally to about 400 mg/day.
- Figure 1 shows that (R)-phenylpiracetam increases extracellular dopamine levels in rat striatum as shown by brain microdialysis.
- Figure 2 shows the concentration of (R)-phenylpiracetam in the brain, as determined by brain microdialysis, after intraperitoneal (i.p.) application. Affinity for DA transporter is approx. 13 ⁇ .
- Figure 3 shows that (R)-phenylpiracetam increases locomotor activity (horizontal activity) in rats (administration i.p. 15 min before test) (*: p ⁇ 0.05 vs. vehicle, Kruskal- Wallis one-way ANOVA on ranks at each time interval followed by rank sum test).
- Figure 4 shows the CNS profile of (R)-phenylpiracetam in an EEG screen.
- Figure 5 shows the results from a progressive ratio test for determining the influence of (R)-phenylpiracetam on motivation in comparison to amphetamine.
- Figure 6 shows the results from a progressive ratio test for determining the influence of (R)-phenylpiracetam on motivation in comparison to methylphenidate.
- Figure 7 shows the results from a cost benefit test for determining the influence of (R)-phenylpiracetam on motivation in comparison to amphetamine.
- Figure 8 shows the results from a cost benefit test for determining the influence of (R)-phenylpiracetam on motivation in comparison to methylphenidate.
- Figure 9 shows the effect of (R)-phenylpiracetam on rotation in rats with unilateral SNc lesion (model of Parkinson's Disease).
- Figure 10 shows the effect of (R)-phenylpiracetam on sedation produced by reserpine (5 mg/kg) + alpha-methyl-p-tyrosine (250 mg/kg)
- Figure 11 shows the effect of (R)-phenylpiracetam on sedation produced by haloperidol (0.2 mg/kg).
- Figure 12 shows the effect of (S)-phenylpiracetam on hypokinesia produced by haloperidol (0.2 mg/kg) (model of Parkinson's Disease) at 50 mg/kg (Fig. 12A) and at 100 mg/kg (Fig. 12B).
- the present invention relates to the use of (R)-phenylpiracetam and any of its salts, solvates and conjugates, which possesses at least an inhibitory activity on the dopamine re-uptake transporter.
- the present invention relates to a method of treating disease-associated fatigue, particularly disease-associated mental fatigue, in a subject in need thereof, comprising the step of administering an effective amount of (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof, for improving such disease-associated fatigue, and to compositions comprising (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof for use in such treatment.
- phenylpiracetam refers to the compound 2-(4- phenyl-2-oxopyrrolidin-1-yl)acetamide (C12H1 4 N2O2; MW 218.3 g/mol). Phenylpiracetam is also known as fenotropil, phenotropyl, fenotropyl, carphedon or phenotropil and was developed in Russia, where it is available as a prescription medicine under the name "Phenotropil ® ". As used herein, phenylpiracetam refers to the substance, as well as its pharmaceutically acceptable salts.
- Phenylpiracetam is optically active and is available as a racemate of two enantiomers, (R)-phenylpiracetam and (S)-phenylpiracetam.
- the International Nonproprietary Name (INN) "Fonturacetam” has been assigned to racemic phenylpiracetam.
- An international patent application with priority date in 2006 first disclosed the separation of the two enantiomers and demonstrated that the (R)- enantiomer is predominantly responsible for the pharmacological activity (WO 2007/104780).
- (R)-Phenylpiracetam showed more pronounced activity in animal models for detecting antidepressant, analgesic, muscle relaxant and psychostimulant effects.
- the patent claims (R)-phenylpiracetam for the use as an antidepressant, as a stress- protective agent, as a modulator of locomotor activity, as a muscle relaxant, and as an analgesic.
- phenylpiracetam exhibits additional pharmacological effects, which are not yet fully identified and are differentiating phenylpiracetam from other pure dopamine reuptake transporter inhibitors.
- phenylpiracetam is administered orally and shows a half-life of 3-5 hours.
- phenylpiracetam is a nootropic drug, which has a pronounced anti-amnesic action, a direct activating effect on the integrative activity of the brain, helps consolidate memory, improves concentration and mental performance, facilitates the learning process, increases the information transfer between the hemispheres of the brain, increases the resistance of brain tissue to hypoxia and toxic effects, has anticonvulsant and anxiolytic effects, regulates the processes of activation and inhibition of central nervous system, and improves mood.
- phenylpiracetam has a positive effect on the metabolism and blood circulation in brain, stimulates the redox processes and increases energy potential through utilization of glucose, improves regional blood flow in ischemic areas of the brain. It increases noradrenaline, dopamine and serotonin content in the brain, does not affect the levels of GABA, associates neither with GABAA nor GABAB receptors, has no noticeable effect on the spontaneous bioelectric activity of the brain, does not influence respiration and the cardiovascular system. It shows no significant diuretic effect and has anorexigenic effect during treatment. According to the Russian package insert, the stimulating effect of phenylpiracetam manifests in the ability to provide a moderate effect on motor responses, to enhance physical performance.
- the moderate psychostimulant effect of the drug is accompanied by an anxiolytic activity, and it improves mood, has some analgesic effect and raises the threshold of pain.
- the adaptogenic effect of phenylpiracetam is manifested in increasing resistance to stress in conditions of excessive mental and physical overload, fatigue, hypokinesia and immobilization, and at low temperatures.
- the term "disease-associated fatigue” refers to pathological fatigue that is independent of the type of fatigue being a normal response to physical exertion or stress (known as peripheral fatigue).
- This peripheral fatigue refers to muscle fatigue and is induced by repetitive muscle contractions (e.g. after athletics sports) (Chaudhuri and Behan, J Neurol Sci. 179 (Suppl. 1 -2), 34-42, 2000; Chaudhuri and Behan, Lancet. 363, 978-988, 2004).
- Disease-orientated fatigue is not caused by muscle overuse or physical impairment outside the central nervous system.
- This central fatigue is a subjective feeling with symptoms of disease-associated mental fatigue (a subjective feeling of having impaired concentration, reduced memory, and speech difficulties) and disease-associated physical fatigue (subjective feeling of being exhausted and lacking energy).
- This subjective disease-associated fatigue can have its origin in particular embodiments by disturbance of the dopaminergic system of the CNS, particularly a lack of dopamine and potentially also homeostatic changes, which lead to an abnormal degree of persistent tiredness, weakness or exhaustion.
- the score on the FSS is at least 4.
- the score on the FSS is at least 4 for at least 2 weeks.
- disease-associated fatigue is fatigue associated or caused by disturbance of the dopaminergic system, particularly a lack of dopamine.
- the term "subject” encompasses mammals including animals and humans.
- treat is used herein to mean to relieve or alleviate at least one symptom of a disease in a subject.
- the term “treat” also denotes to arrest, delay the onset (i.e., the period prior to clinical manifestation of a disease) and/or reduce the risk of developing or worsening a disease.
- treatment includes modifying, curative and symptomatic treatments.
- terapéuticaally effective applied to dose or amount refers to that quantity of a compound or pharmaceutical composition sufficient to result in a desired activity upon administration to a mammal in need thereof.
- compositions of the invention refers to molecular entities and other ingredients of such compositions that are physiologically tolerable and do not typically produce untoward reactions when administered to a mammal (e.g., human).
- pharmaceutically acceptable may also mean approved by a regulatory agency of the federal or a state government or listed in the U.S. Pharmacopeia or other generally recognized pharmacopeia for use in mammals, and more particularly in humans.
- salt is defined as a chemical containing different charged components.
- salt also includes hydrates and solvates.
- (R)-Phenylpiracetam may be used according to the invention in the form of any of pharmaceutically acceptable salts, solvates and conjugates. Any references to (R)- phenylpiracetam in this description should be understood as also referring to such salts, solvates and conjugates.
- (R)-phenylpiracetam is present as (R)-phenylpiracetam hydrochloride.
- the disease-associated fatigue is a fatigue selected from the list of: fatigue in PD, fatigue in cancer, fatigue post-stroke, fatigue in burn-out syndrome, fatigue in multiple sclerosis, fatigue in HIV/AIDS and other infectious diseases of the CNS, fatigue in fibromyalgia, fatigue in sarkoidosis, fatigue in rheumatic disorders, fatigue in muscle dystrophies, fatigue in lupus erythematosus, fatigue in Morbus Crohn, fatigue in spondylitis ankylosans (Morbus Bechterew), fatigue in pulmonary arterial hypertension, fatigue in depression, fatigue in dementia, chronic fatigue syndrome, fatigue in chronic intoxication, fatigue in traumatic brain injury, fatigue in hypoxic brain damage, fatigue in hepatic infections, e.g.
- hepatitis C fatigue in cirrhosis, e.g. primary biliary cirrhosis, fatigue in cachexia (age and tumor related), fatigue in amyotrophic lateral sclerosis (ALS), post-polio fatigue, and fatigue in myasthenia gravis.
- cirrhosis e.g. primary biliary cirrhosis
- cachexia age and tumor related
- ALS amyotrophic lateral sclerosis
- post-polio fatigue fatigue in myasthenia gravis.
- the disease-associated fatigue is fatigue in Parkinson's disease.
- the present invention relates to a method of treating fatigue-associated symptoms in PD, particularly inactivity, motivational-deficit, floppiness, exhaustion, lassitude, and prostration.
- Parkinson Fatigue Scale A well-accepted measurement of this type of fatigue is the Parkinson Fatigue Scale, which has been described above.
- the disease-associated fatigue is mental fatigue in Parkinson's disease.
- the score on the Parkinson Fatigue Scale is at least 7.
- the score on the Parkinson Fatigue Scale is at least 7 for at least 2 weeks.
- the disease-associated fatigue is fatigue in cancer.
- the disease-associated fatigue is mental fatigue in cancer.
- the present invention relates to a method of treating sleep-associated problems of PD patients, which have significant impact on their quality of life, particularly general tiredness, and drowsiness.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 1 mg to about 400 mg/day.
- the term “about” or “approximately” means between 90% and 1 10% of a given value or range, i.e. "about 100" means “between 90 and 1 10". In narrower embodiments, the term “about” or “approximately” means between 95% and 105% of a given value or range, or between 98% and 102% of a given value or range, or between 99% and 101 % of a given value or range. [0084] In a further embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 25 mg to about 350 mg/day.
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 50 mg to about 300 mg/day, particularly in a range from about 50 mg to about 150 mg/day.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered once a day, particularly at a dose of about 200 mg once a day.
- (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a multiple dose, for example twice a day (bid.), or three times a day, particularly twice a day, particularly at a dose of about 100 mg twice a day.
- compositions comprising a therapeutically effective amount of (R)- phenylpiracetam.
- the compositions of the invention may further comprise a carrier or excipient (all pharmaceutically acceptable).
- the compositions may be formulated e.g. for once-a-day administration, twice-a-day administration, or three times a day administration.
- carrier applied to pharmaceutical compositions of the invention refers to a diluent, excipient, or vehicle with which an active compound (e.g., (R)- phenylpiracetam) is administered.
- Such pharmaceutical carriers may be sterile liquids, such as water, saline solutions, aqueous dextrose solutions, aqueous glycerol solutions, and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like. Suitable pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences” by A.R. Gennaro, 20 th Edition.
- the active ingredient e.g.
- (R)-phenylpiracetam) or the composition of the present invention may be used for the treatment of at least one of the mentioned disorders, wherein the treatment is adapted to or appropriately prepared for a specific administration as disclosed herein (e.g., to once-a-day, twice-a-day, or three times a day administration).
- the package leaflet and/or the patient information contains corresponding information.
- the active ingredient e.g., (R)-phenylpiracetam
- the composition of the present invention may be used for the manufacture of a medicament for the treatment of at least one of the mentioned disorders, wherein the medicament is adapted to or appropriately prepared for a specific administration as disclosed herein (e.g., to once-a- day, twice-a-day, or three times a day administration).
- the package leaflet and/or the patient information contains corresponding information.
- the dosage form of (R)-phenylpiracetam, or a (R)-phenylpiracetam salt may be a solid, semisolid, or liquid formulation.
- (R)-Phenylpiracetam may be administered via different application routes.
- the oral and the parenteral route are the preferred routes of application.
- (R)- Phenylpiracetam may be formulated as a flavored liquid, a capsule or a tablet.
- (R)-phenylpiracetam may be combined with non-toxic, pharmaceutically acceptable excipients.
- the optimal therapeutically effective amount may be determined experimentally, taking into consideration the exact mode of administration, form in which the drug is administered, the indication toward which the administration is directed, the subject involved (e.g., body weight, health, age, sex, etc.), and the preference and experience of the physician or veterinarian in charge.
- Suitable daily doses of the active ingredient of the invention in therapeutic treatment of humans are within the range from about 1 mg to about 400 mg per day (based on (R)-phenylpiracetam as free base), such as from about 25 mg to about 350 mg, or from about 50 mg to about 300 mg, particularly about 200 mg per day.
- the daily dose may be body weight-adjusted such as about 200 mg/day up to 80 kg body weight or 240 mg/day for patients with a body weight of ⁇ 80 kg.
- (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from starting from about 50 mg and increasing the dose in 50 mg steps until the desired therapeutic efficacy is reached, but maximally to about 400 mg/day.
- the total amount of active ingredient per day of administration could also be higher due to reduced bioavailability, e.g. up to about 500 mg/day.
- a pharmaceutically acceptable salt, a solvate, a conjugate or a derivative of (R)-phenylpiracetam, such as (R)-phenylpiracetam hydrochloride the corresponding amount may be adjusted so that an equimolar amount is used.
- (R)-Phenylpiracetam may be administered as a single anti-fatigue agent or in combination with one or more additional pharmaceutical agents for the therapy of fatigue, particularly for the treatment of mental fatigue.
- said one or more additional pharmaceutical agents are selected from rasagiline and pramipexole.
- the pharmaceutical composition comprising (R)- phenylpiracetam may further comprise at least one additional active agent as defined herein, particularly wherein the additional active agent is for the treatment of the disease the fatigue is associated with.
- the disease is Parkinson's disease (PD).
- (R)-phenylpiracetam is administered in combination with a drug, which increases the tolerability of the treatment with (R)- phenylpiracetam and/or reduces at least one side effect associated with the treatment with (R)-phenylpiracetam.
- Recombinant Chinese hamster ovary (CHO) cells CHO-K1 (ATCC ® CCL- 61TM) cells stably expressing dopamine transporters are plated.
- the cells (2 x 10 5 /ml) are pre-incubated with test compound and/or vehicle in modified Tris-HEPES buffer pH 7.1 at 25°C for 20 min and 50 nM [ 3 H]-dopamine is then added for an additional 15 min incubation period.
- Non-specific signal is determined in the presence of 10 ⁇ nomifensine.
- Cells are then solubilized with 1 % SDS lysis buffer.
- Reduction of [ 3 H]- dopamine uptake by 50 percent or more (> 50%) relative to vehicle controls indicates significant inhibitory activity.
- Compounds are screened at 10, 1 , 0.1 , 0.01 and 0.001 ⁇ . These same concentrations are concurrently applied to a separate group of untreated cells and evaluated for possible compound-induced cytotoxicity only if significant inhibition of
- Recombinant Madin Darby canine kidney (MDCK) cells NBL-2) (ATCC ® CCL-34TM) expressing norepinephrine transporter are plated for two days.
- Test compound and/or vehicle is pre-incubated with cells (1 x 10 5 /ml) in modified Tris- HEPES buffer pH 7.1 for 20 min at 25°C and 25 nM [ 3 H]-norepinephrine is then added for an additional 15 min incubation period.
- a lysate is obtained from solubilized cells and counted to determine [ 3 H]-norepinephrine uptake.
- DA Dopamine
- DOPAC 3,4-Dihydroxyphenylacetic acid
- Chromatographic separation was performed using a mobile phase that consisted of a sodium acetate buffer (6.15 g/l) with methyl alcohol (2.5% v/v), Titriplex (250 mg/l), 1-octanesulfonic acid (OSA, 150 mg/l), and adjusted with glacial acetic acid to pH 4.1 (isocratic).
- the mobile phase was run through the system at a flow rate of 0.35 ml/min by an HPLC pump (Shimadzu, model LC-10AD vp, Japan).
- DA and DOPAC Concentrations of DA and DOPAC were determined in the same sample, by HPLC separation and electrochemical detection. DA and DOPAC were detected electrochemically using a potentiostate (Antec Leyden, model Intro, the Netherlands) fitted with a glassy carbon electrode set at +500 mV vs. Ag/AgCI (Antec Leyden, the Netherlands). Data was analyzed by Chromatography Data System (Shimadzu, class- p, Japan) software. Concentrations were quantified by the external standard method.
- Siliconized guide cannula (MAB 6.14.IC) (MAB, Sweden) were implanted unilaterally in pentobarbital anaesthetized animals aiming at the caudatus putamen (CPu; AP: +0.1 , LM: ⁇ 2.6, DV: -3.2 mm relative to bregma; -3.3 mm interaural) according to the atlas of Paxinos and Watson (loc. cit.). Rats were given at least 3 days to recover from surgery before starting microdialysis experiments.
- Microdialysis experiments were performed in the home cage of the animal.
- a microdialysis probe (MAB 6.14.4.; 4 mm exposed membrane length, polyethersulfone (PES) membrane; MAB, Sweden) was lowered through the guide cannula into the CPu (ventral position of probe tip with reference to the skull: -7.2 mm) ca. 12 hours before the sampling and left in place for the whole testing period.
- PES polyethersulfone
- the probes were perfused with aCSF at a flow rate of 2 ⁇ /min using a CMA 102 perfusion pump (CMA, Solna, Sweden).
- the composition of the aCSF was 147 mM Na + , 2.7 mM K + , 1.2 mM Ca 2+ , 0.85 mM Mg 2+ , 0.04 mM ascorbic acid.
- the animals were connected by a head block tether system (Instech, Plymouth Meeting, USA) to a dual channel liquid swivel 375/D/22QM (Instech, Plymouth Meeting, USA). FEP tubing and tubing adapters (MAB, Sweden) were used.
- the sample collection began one hour after start of perfusion with three 20-minutes fractions (baseline). Thereafter, each rat was injected i.p. with (R)-phenylpiracetam and/or L- DOPA (25 mg/kg + benserazide, 15 mg/kg).
- the samples (40 pi) were collected automatically with a fraction collector (CMA/142; CMA, Solna, Sweden) and stored at - 20°C until analysis.
- probes were inserted into a beaker with aCSF (37°C) containing 100 nM solution of (R)-phenylpiracetam or L-DOPA.
- Five samples (40 ⁇ ) were collected while only the last 2 were used for analysis of (R)- phenylpiracetam or L-DOPA concentration.
- HPLC in combination with atmospheric pressure ionization tandem mass spectrometry (API-MS/ S) was employed (HPLC (Shimadzu Prominence, Duisburg, Germany) coupled to an API 4000 Q Trap (triple quadrupole, Applied Biosystems/MDS Sciex, Darmstadt, Germany) equipped with a Turbolonspray source (ESI)).
- the analytical column was a Onyx Monolithic C18 50 mm x 2 mm (Phenomenex, Aillesburg, Germany).
- the mobile phase consisted of the eluent A water and eluent B acetonitrile both containing 0.1 % of formic acid.
- the chromatographic run consisted of a gradient over 1.5 min from 5% acetonitrile in water at start until a mobile phase composition of 50% acetonitrile in water. A volume of 1 ml was injected in the API/MS/MS. A standard curve was used to calculate sample concentrations of studied agents in our samples.
- Locomotor activity was measured in 8 perspex boxes (ENV-515-16, 43.2 mm x 43.2 mm x 30 cm), Med-Associates Inc.) equipped with 4 arrays of 16 infrared photobeams placed 3 cm above the floor measure horizontal activity. Distance traveled (DT) was used in further analysis as a measure of locomotion.
- Rats were implanted with 4 bipolar concentric steel electrodes within a stereotactic surgical procedure during anaesthesia with ketamine. All four electrodes were placed 3 mm lateral within the left hemisphere. Dorsoventral coordinates were 4, 6, 4.2 and 8 mm and anterior coordinates were 3.7, 9.7, 5.7 and 12.2 mm for frontal cortex, striatum, hippocampus, and reticular formation, respectively (according to the atlas of Paxinos and Watson, 1982).
- a p re-constructed base plate carrying 4 bipolar stainless steel semi-micro electrodes (neurological electrodes "SNF 100" from Rhodes Medical Instruments, Inc., Summerland, CA 93067, USA) and a 5-pin-plug was fixed to the skull by dental cement interacting with 3 steel screws placed on distance into the bone.
- the distant recording spot of the electrode was the active electrode whereas the proximal spots of the four electrodes were connected to each other to give a reference.
- the base plate was carrying a plug to receive later on the transmitter (weight: 5.2 g including battery, 26 mm x 12 mm x 6 mm of size).
- Electroencephalographic (EEG) signals were recorded from frontal cortex, hippocampus, striatum and reticular formation of freely moving rats from inside a totally copper shielded room. Signals were wirelessly transmitted by a radio-telemetric system (Rhema Labortechnik, Hofheim, Germany, using 40 MHz as carrier frequency) and were amplified and processed as described earlier to give power spectra of 0.25 Hz resolution (Dimpfel et al. 1986; Dimpfel et al. 1988; Dimpfel et al. 1989; Dimpfel, 2003). In short, after automatic artifact rejection signals were collected in sweeps of 4 s duration and fast-fourier transformed using a Hanning window. Sampling frequency was 512 Hz.
- (R)-Phenylpiracetam produced a dose dependent attenuation of alpha2 and betal waves (see Figure 4). The highest dosage produced a different pattern of changes in that theta power increases within the frontal cortex were observed. Motility was increased. Alpha2 waves are mainly under the control of dopamine (Dimpfel, loc. cit.). Thus, direct or indirect effects on dopaminergic neurotransmission can be expected from (R)-phenylpiracetam. Attenuation of alpha2 and betal waves has also been observed after administration drugs used for treatment of M. Parkinson (Dimpfel and Hoffmann, Neuropsychobiology, 62, 213-220, 2010). In summary (R)- phenylpiracetam shows a profile within the area of stimulatory drugs.
- Methylphenidate purchased from Sigma (Taufkirchen, Germany) was dissolved in distilled water fresh for each test day.
- Modafinil purchased from Sequoia Research Products Limited (Pangbourne, UK) was dissolved in 1% w/v methylcellulose (Sigma, Taufmün, Germany) in 0.9% NaCI water fresh for each test day.
- (R)- phenylpiracetam was dissolved in distilled water fresh for each test day.
- Test 1 Progressive ratio (PR) task
- Behavioral testing was conducted in 12 operant test chambers (Med Associates, St. Albans, USA). Each chamber was equipped with a retractable lever, a food dispenser with receptacle, an overhead house light and two stimulus lights, one above the lever and the other above the food receptacle. An infrared photocell beam detected nose pokes into the food receptacle.
- the apparatus was controlled by a computer system (SmartControl®-lnterface and Med PC-software, Med Associates, St. Albans, USA). The light above the food receptacle indicated the delivery of a food pellet in the receptacle. Rats were first habituated to the operant boxes for two sessions (30 min each) on two consecutive days.
- a session lasted for 90 min or was ended when a rat failed to press the lever for consecutive 10 min.
- the value of the last completed ratio (breaking point) was recorded as well as the amount of received rewards, the perseverative lever presses, the duration of the session and the latency to respond.
- Animals of all treatment groups received respective vehicle/drug infusions 30 min pre-test on the final two sessions involving testing under a PR schedule.
- the rats had the choice between working for their preferred food (Bioserve pellets) by pressing the lever on a PR schedule as described above or obtaining lab chow being freely available in a dish (about 15 g) within the operant chamber.
- the food receptacle and the lever were positioned on the same wall of the operant chamber, the dish containing the lab chow was situated in a corner on the opposite side of the operant chamber.
- a session lasted for 90 min or ended when a rat failed to press the lever for consecutive 10 min. Thereafter the amount of lab chow ingested was calculated. Animals of all treatment groups received vehicle/drug infusions 30 min pre-test.
- (R)-Phenylpiracetam was tested in rats with unilateral SNc lesions - a preclinical model of Parkinson's disease. In this model, ipsilateral rotations indicate a presynaptic mode of action, which is consistent with inhibition of dopamine uptake as a primary mode of action.
- Rats were injected with substance, placed in Perspex cylinders (30 cm diameter), and the rotational behaviour (360°) was scored up to 360 min at 20-min intervals using TSA rotation measurement system.
- the locomotor activity was measured in four perspex boxes (ENV-515-16, 43.2 cm x 43.2 cm x 30 cm), Med-Associates Inc. system) equipped with 4 arrays of 16 infrared photobeams placed 3 cm above the box floor. Distance travelled (DT) was used in further analysis for measuring locomotion. The recording started immediately after placing animals in the open field.
- the locomotor activity was measured in four perspex boxes (ENV-515-16, 43.2 cm x 43.2 cm x 30 cm), Med-Associates Inc. system) equipped with 4 arrays of 16 infrared photobeams placed 3 cm above the box floor. Distance travelled (DT) was used in further analysis for measuring locomotion. The recording started immediately after placing animals in the open field.
Abstract
The present invention relates to the efficient treatment of an individual afflicted with fatigue, particularly mental fatigue, associated with certain diseases, particularly diseases of the central nervous system, including Parkinson's disease (PD), the instant treatment comprising administering to the individual an effective amount of (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof.
Description
USE OF (R)-PHENYLPIRACETAM FOR THE TREATMENT OF
DISEASE-ASSOCIATED FATIGUE
FIELD OF THE INVENTION
[0001] The present invention relates to the efficient treatment of an individual afflicted with fatigue, particularly mental fatigue, associated with certain diseases, particularly diseases of the central nervous system, including Parkinson's disease (PD), the instant treatment comprising administering to the individual an effective amount of (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof.
BACKGROUND OF THE INVENTION
[0002] This invention relates to a method of treating patients afflicted with certain forms of fatigue associated with diseases, particularly of the central nervous system (CNS), including Parkinson's disease.
[0003] Fatigue can be experienced both as physical fatigue, affecting the muscles and/or the ability to move, and as mental fatigue, affecting the level of attention, motivation and/or consciousness.
[0004] In many debilitating diseases, particularly including diseases affecting the CNS, the development of a disease-associated fatigue can be observed. Examples include fatigue in PD, fatigue in cancer, fatigue post-stroke, fatigue in burn-out syndrome, fatigue in multiple sclerosis, fatigue in HIV/AIDS and other infectious diseases of the CNS, fatigue in fibromyalgia, fatigue in sarkoidosis, fatigue in rheumatic disorders, fatigue in muscle dystrophies, fatigue in lupus erythematosus, fatigue in Morbus Crohn, fatigue in spondylitis ankylosans (Morbus Bechterew), fatigue in pulmonary arterial
hypertension, fatigue in depression, fatigue in dementia, chronic fatigue syndrome, fatigue in chronic intoxication, fatigue in traumatic brain injury, fatigue in hypoxic brain damage, fatigue in hepatic infections, e.g. hepatitis C, fatigue in cirrhosis, e.g. primary biliary cirrhosis, fatigue in cachexia (age and tumor related), fatigue in amyotrophic lateral sclerosis (ALS), post-polio fatigue, and fatigue in myasthenia gravis. Affected patients experience fatigue either constantly without any relevant physical or mental activity or already after a short or slight effort. That kind of pathological fatigue has significant impact on patients' quality of life and calls for therapy.
[0005] Disease-associated fatigue, as further defined below, has to be differentiated from fatigue symptoms in healthy individuals, where fatigue is a normal result of a natural reaction of body and mind to long-lasting and/or heavy burden, working, mental stress, over-stimulation or under-stimulation, jet lag, boredom, or lack of sleep. The physiological fatigue in healthy individuals is a normal response to physical exertion or stress, and its function is simply to protect the body from damage by overcharge. Such type of fatigue normally disappears spontaneously after a short recovery period. This type of fatigue isn't pathological and needs no therapeutic intervention. It is excluded from the intended medical use of the present invention.
[0006] Only the pathological type of fatigue is targeted by the present invention, and, unless otherwise defined, the term "fatigue" as used herein stands for the pathological form only.
[0007] A well-accepted measurement of this type of fatigue is the Fatigue Severity Scale (FSS), a self-administered unidimensional generic 9-item fatigue rating scale (Krupp et al., Arch Neurol. 46, 1 121-23, 1989). Each item has to be rated on a seven-grade Likert scale with a range from 1 (completely disagree) to 7 (completely agree), see Table 1 . The total FSS score is mean score of the scores on the respective 9 items. A total FSS score of 4 or higher present over 2 weeks prior to scoring is accepted as definition of presence of disease-orientated fatigue, especially in chronic diseases, e.g. Parkinson's disease.
Table 1 : Fatigue Severity Scale
[0008] Cancer-related fatigue is defined as a persistent, subjective sense of (i) physical, and/or (ii) emotional and/or cognitive (mental) tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and that significantly interferes with usual functioning (National Comprehensive Cancer Network (NCCN) guidelines). Cancer-related fatigue differs from the fatigue that accompanies everyday life, which is usually temporary and relieved by rest.
[0009] Parkinson's disease (PD) is one of the most common chronic neurological diseases. The classical symptoms of PD are rigor (muscular stiffness), tremor and bradykinesia (slowness of movements). These movement disturbances can nowadays be treated rather effectively with various available PD drugs, particularly in early stages of the disease. However, in recent years it has been increasingly recognized that the current challenge of PD treatment are the so-called "non-motor" symptoms, for which satisfactory treatments are mainly lacking (Gallagher et al., Mov Disord. 2010 Nov 15;25(15):2493-500). Amongst non-motor symptoms are autonomic dysfunctions (cardiovascular, urinary and gastrointestinal), sleep problems, psychosis, pain, cognitive deficits and fatigue. In clinical studies it could be demonstrated that fatigue and depression have the strongest association with a decline in the quality of PD patients' life (Beiske et al. Mov Disord. 2010 Oct 30;25(14):2456-60; Beiske and Svensson, Acta Neurol Scand Suppl. 2010;(190):78-81).
[00 0] Fatigue in PD is multidimensional including physical, mental and general aspects (Havlikova et al., Parkinsonism Relat Disord. 14, 187-92, 2008; Havlikova et al., Eur J Neurol. ;15(5):475-80, 2008; Havlikova et al., J Neurol Sci. 270, 107-13, 2008). The physical dimensions of fatigue in PD are connected to problems regarding mobility and activity of daily living. Mental fatigue dimensions affect cognition, emotional well-being, and communication. In addition, general fatigue is related to bodily discomfort of the patients.
[0011] Treatment options for fatigue in PD are rather limited. Recently studies with approved PD drugs have been published that showed besides the main effects on motor symptoms also mediocre improvement of fatigue. Those drugs are the monoamine oxidase inhibitor rasagiline (Rascol et al., Lancet Neurol. 10, 415-423, 2011) and the dopamine agonist pramipexole (Morita et al., Intern Med. 50, 2163-2168, 2011). However, a specific drug with a strong anti-fatigue effect that is well tolerated by PD patients is so far not available.
[0012] Karabanov et al. describe an open-label study of the effects of phenylpiracetam on patients suffering from PD (Karabanov et al., Atmosfera. Nervnye Bolezni 4, 29-32, 2009). In the study report, it is mentioned that fatigue was one of the parameters being monitored in the study, and a figure is presented showing a positive effect of phenylpiracetam on the combined parameter "general activity, physical and mental fatigue, asthenization symptoms". Karabanov neither presents data on the effect of phenylpiracetam on fatigue alone, nor differentiates between disease-related fatigue and "physiological" fatigue. Similarly, Kalinskij and Nazarov, describe the effect of phenylpiracetam on fatigability in the treatment of asthenic syndrome (Kalinskij and Nazarov, Zh Nevrol Psikhiatr Im SS Korsakova, 107, 61-63, 2007), and Akhapkina et al. provide additional data from a clinical study Efficacy of Phenotropil in the treatment of asthenic syndrome and chronic fatigue syndrome (Akhapkina et al., Atmosfera. Nervnye Bolezni 3, 28-31 , 2004). Karabanov et al. are stressing "asthenization", which is a condition experienced by astronauts (healthy subjects) following long-term space flights, in which following return to Earth the astronaut experiences symptoms such as fatigue, irritability, lack of appetite and sleep disorders. Interestingly, this is derived from
the original use of the compound as "cosmic drug" (Mendonca et al, Mov. Disord. 22, 2070-2076, 2007).
[0013] In contrast, the so-called "Parkinson Fatigue" is an outstanding embodiment of disease-associated fatigue seen exclusively in PD patients. Using common rating scales for fatigue causes problems in PD patients, as the critical assessment of Friedman JH et al (Mov. Disorders, 25, 805-822, 2010) revealed. The "Parkinson Fatigue Scale" developed by Brown RG et al. (Parkinsonism Related Disorders 2005, 1 1 (1):49-55) excludes emotional and cognitive features that may occur as part of the fatigue experience but which may also occur independently in Parkinsonism. The 16 specific items of this scale are reported by the affected patients themselves and allow a clear separation of the Parkinson-specific type of fatigue from other types of fatigue, wherein the cut-off point is at 7 (binary calculation). However, the Parkinson Fatigue Score is not ideally suited for analyzing mental fatigue associated with Parkinson's disease alone.
[0014] Thus, despite the fact that many attempts have been made to develop agents for the treatment of disease-associated fatigue, so far these attempts have had no clinically meaningful success in patients.
[0015] Thus, there is still a large unmet need to identify medicaments for the treatment of disease-associated fatigue, particularly for the treatment of disease-associated mental fatigue.
[0016] The solution provided by the present invention to solve this problem, i.e. the use of a particular compound, has so far not been achieved or suggested by the prior art.
SUMMARY OF THE INVENTION
[0017] The present invention relates to a method of treating disease-associated fatigue, particularly disease-associated mental fatigue, in a subject in need thereof, comprising the step of administering an effective amount of (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof.
[0018] In certain embodiments of the method of the present invention, (R)- phenylpiracetam is present as (R)-phenylpiracetam hydrochloride.
[0019] In certain embodiments, the disease-associated fatigue is fatigue in Parkinson's disease.
[0020] In certain embodiments, the disease-associated fatigue is mental fatigue in Parkinson's disease.
[0021] In certain embodiments, the disease-associated fatigue is fatigue in cancer.
[0022] In certain embodiments, the disease-associated fatigue is mental fatigue in cancer.
[0023] In certain embodiments of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 1 mg to about 400 mg/day.
[0024] In a further embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 25 mg to about 350 mg/day.
[0025] In a still further embodiment, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 50 mg to about 300 mg/day.
[0026] In yet another embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered once a day, particularly at a dose of about 200 mg once a day.
[0027] In yet another embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a
multiple dose, for example twice a day (b.i.d.), or three times a day, particularly twice a day, particularly at a dose of about 100 mg twice a day.
[0028] In a still further embodiment, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from starting from 50 mg and increasing the dose in 50 mg steps until the desired therapeutic efficacy is reached, but maximally to 400 mg/day.
[0029] In yet another embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in an oral formulation.
[0030] In another aspect, the present invention relates to a pharmaceutical composition comprising (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof for use in the treatment of disease-associated fatigue.
[0031] In certain embodiments of the pharmaceutical composition of the present invention, (R)-phenylpiracetam is present as (R)-phenylpiracetam hydrochloride.
[0032] In certain embodiments of the pharmaceutical composition of the present invention, the treatment comprises administering (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof in a range from about 1 mg to about 400 mg/day.
[0033] In a further embodiment of the pharmaceutical composition of the present invention, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in a range from about 25 mg to about 350 mg/day.
[0034] In a still further embodiment, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in a range from about 50 mg to about 300 mg/day, particularly in a range from about 50 mg to about 150 mg/day.
[0035] In yet another embodiment of the pharmaceutical composition of the present invention, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof (e.g. , (R)- phenylpiracetam hydrochloride) is for administration once a day, particularly about 200 mg once a day, twice a day (b.i.d.), particularly at a dose of about 100 mg twice a day, or three times a day, particularly once or twice a day.
[0036] In a still further embodiment, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from starting from about 50 mg and increasing the dose in 50 mg steps until the desired therapeutic efficacy is reached, but maximally to about 400 mg/day.
[0037] In yet another embodiment of the pharmaceutical composition of the present invention, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof for administration in an oral formulation.
BRIEF DESCRIPTION OF THE DRAWINGS
[0038] Figure 1 shows that (R)-phenylpiracetam increases extracellular dopamine levels in rat striatum as shown by brain microdialysis.
[0039] Figure 2 shows the concentration of (R)-phenylpiracetam in the brain, as determined by brain microdialysis, after intraperitoneal (i.p.) application. Affinity for DA transporter is approx. 13 μΜ.
[0040] Figure 3 shows that (R)-phenylpiracetam increases locomotor activity (horizontal activity) in rats (administration i.p. 15 min before test) (*: p < 0.05 vs. vehicle, Kruskal- Wallis one-way ANOVA on ranks at each time interval followed by rank sum test).
[0041 ] Figure 4 shows the CNS profile of (R)-phenylpiracetam in an EEG screen.
[0042] Figure 5 shows the results from a progressive ratio test for determining the influence of (R)-phenylpiracetam on motivation in comparison to amphetamine.
[0043] Figure 6 shows the results from a progressive ratio test for determining the influence of (R)-phenylpiracetam on motivation in comparison to methylphenidate.
[0044] Figure 7 shows the results from a cost benefit test for determining the influence of (R)-phenylpiracetam on motivation in comparison to amphetamine.
[0045] Figure 8 shows the results from a cost benefit test for determining the influence of (R)-phenylpiracetam on motivation in comparison to methylphenidate.
[0046] Figure 9 shows the effect of (R)-phenylpiracetam on rotation in rats with unilateral SNc lesion (model of Parkinson's Disease).
[0047] Figure 10 shows the effect of (R)-phenylpiracetam on sedation produced by reserpine (5 mg/kg) + alpha-methyl-p-tyrosine (250 mg/kg)
[0048] Figure 11 shows the effect of (R)-phenylpiracetam on sedation produced by haloperidol (0.2 mg/kg).
[0049] Figure 12 shows the effect of (S)-phenylpiracetam on hypokinesia produced by haloperidol (0.2 mg/kg) (model of Parkinson's Disease) at 50 mg/kg (Fig. 12A) and at 100 mg/kg (Fig. 12B).
DETAILED DESCRIPTION OF THE INVENTION
[0050] The peculiarity of this invention compared to former treatment approaches for treating disease-associated fatigue, particularly disease-associated mental fatigue, is the so far unknown therapeutic efficacy of (R)-phenylpiracetam, which is presumably based at least in part on the newly identified activity of (R)-phenylpiracetam as the dopamine re-uptake inhibitor.
[0051] Thus, the present invention relates to the use of (R)-phenylpiracetam and any of its salts, solvates and conjugates, which possesses at least an inhibitory activity on the dopamine re-uptake transporter.
[0052] Thus, in a particular aspect the present invention relates to a method of treating disease-associated fatigue, particularly disease-associated mental fatigue, in a subject in need thereof, comprising the step of administering an effective amount of (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof, for improving such disease-associated fatigue, and to compositions comprising (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof for use in such treatment.
[0053] The term "phenylpiracetam" is known in the art and refers to the compound 2-(4- phenyl-2-oxopyrrolidin-1-yl)acetamide (C12H14N2O2; MW 218.3 g/mol). Phenylpiracetam is also known as fenotropil, phenotropyl, fenotropyl, carphedon or phenotropil and was developed in Russia, where it is available as a prescription medicine under the name "Phenotropil®". As used herein, phenylpiracetam refers to the substance, as well as its pharmaceutically acceptable salts.
[0054] Phenylpiracetam is optically active and is available as a racemate of two enantiomers, (R)-phenylpiracetam and (S)-phenylpiracetam. The International Nonproprietary Name (INN) "Fonturacetam" has been assigned to racemic phenylpiracetam. An international patent application with priority date in 2006 first disclosed the separation of the two enantiomers and demonstrated that the (R)- enantiomer is predominantly responsible for the pharmacological activity (WO 2007/104780). (R)-Phenylpiracetam showed more pronounced activity in animal models for detecting antidepressant, analgesic, muscle relaxant and psychostimulant effects. The patent claims (R)-phenylpiracetam for the use as an antidepressant, as a stress- protective agent, as a modulator of locomotor activity, as a muscle relaxant, and as an analgesic.
[0055] More detailed pharmacological data with (R)-phenylpiracetam have been published recently by the same authors (Zvejniece et al., Basic Clin Pharmacol Toxicol. 109, 407-12, 201 1 ). In the open-field test a significant increase in locomotor activity was found, which was just slightly stronger with the (R)-phenylpiracetam compared to (S)- phenylpiracetam. Also in the forced swim test, used as a model for depression, (R)- phenylpiracetam was also only slightly more potent than (S)-phenylpiracetam. However, in the passive avoidance test, (R)-phenylpiracetam enhanced memory function
significantly better than (S)-phenylpiracetam. The authors conclude that these results may be important for the clinical use of optically pure isomers of phenylpiracetam.
[0056] Prior to the surprising finding of the present inventors that (R)-phenylpiracetam acts as a dopamine re-uptake transporter inhibitor, the precise pharmacological mechanism of action of phenylpiracetam had not been elucidated. In pharmacological studies phenylpiracetam was found to activate the operant behaviour, to counteract psychodepressant effects of diazepam, to inhibit post-rotational nystagmus, and to prevent the development of retrograde amnesia. It also exhibited anticonvulsant action (Bobkov et al., Biull Eksp Biol Med. 95, 50-53, 1983) and some neuroprotective activity in experimental cerebral ischemia (Tiurenkov et al., Eksp Klin Farmakol., 70, 24-29, 2007). Thus, phenylpiracetam exhibits additional pharmacological effects, which are not yet fully identified and are differentiating phenylpiracetam from other pure dopamine reuptake transporter inhibitors.
[0057] In humans phenylpiracetam is administered orally and shows a half-life of 3-5 hours. There are only a small number of low-scale exploratory clinical trials predominantly published in Russian journals. They have shown possible links between intake of phenylpiracetam and improvement in a number of conditions and diseases including asthenic syndrome and autonomic disturbances in brain trauma (Kalinskij and Nazarov, Zh Nevrol Psikhiatr Im S S Korsakova 107, 61-63, 2007), brain organic lesions (Savchenko et al. Zh Nevrol Psikhiatr Im S S Korsakova., 105, 22-26, 2005), epilepsy (Bel'skaia et al. Zh Nevrol Psikhiatr Im S S Korsakova 107, 40-43, 2007; Lybzikova et al., Zh Nevrol Psikhiatr Im S S Korsakova.108, 69-70, 2008), stomatological problems (Novikova et al. Stomatologiia (Mosk).87, 41-45, 2008), and vascular encephalopathy (Gustov et al. Zh Nevrol Psikhiatr Im S S Korsakova.106, 52-53, 2006). Effects of phenylpiracetam on immunological consequences of stroke have also been described (Gerasimova et al., Zh Nevrol Psikhiatr Im S S Korsakova, 105, 63-64, 2005).
[0058] According to the package insert of the drug as approved in Russia, phenylpiracetam is a nootropic drug, which has a pronounced anti-amnesic action, a direct activating effect on the integrative activity of the brain, helps consolidate memory, improves concentration and mental performance, facilitates the learning process,
increases the information transfer between the hemispheres of the brain, increases the resistance of brain tissue to hypoxia and toxic effects, has anticonvulsant and anxiolytic effects, regulates the processes of activation and inhibition of central nervous system, and improves mood. It is furthermore stated that phenylpiracetam has a positive effect on the metabolism and blood circulation in brain, stimulates the redox processes and increases energy potential through utilization of glucose, improves regional blood flow in ischemic areas of the brain. It increases noradrenaline, dopamine and serotonin content in the brain, does not affect the levels of GABA, associates neither with GABAA nor GABAB receptors, has no noticeable effect on the spontaneous bioelectric activity of the brain, does not influence respiration and the cardiovascular system. It shows no significant diuretic effect and has anorexigenic effect during treatment. According to the Russian package insert, the stimulating effect of phenylpiracetam manifests in the ability to provide a moderate effect on motor responses, to enhance physical performance. The moderate psychostimulant effect of the drug is accompanied by an anxiolytic activity, and it improves mood, has some analgesic effect and raises the threshold of pain. The adaptogenic effect of phenylpiracetam is manifested in increasing resistance to stress in conditions of excessive mental and physical overload, fatigue, hypokinesia and immobilization, and at low temperatures.
[0059] Thus, while phenylpiracetam has apparently been prescribed inter alia for the treatment of stress associated with fatigue in healthy patients, phenylpiracetam has hitherto not been associated with the treatment of fatigue as such, neither non- pathological nor pathological fatigue.
[0060] In the context of the present invention, the term "disease-associated fatigue" refers to pathological fatigue that is independent of the type of fatigue being a normal response to physical exertion or stress (known as peripheral fatigue). This peripheral fatigue refers to muscle fatigue and is induced by repetitive muscle contractions (e.g. after athletics sports) (Chaudhuri and Behan, J Neurol Sci. 179 (Suppl. 1 -2), 34-42, 2000; Chaudhuri and Behan, Lancet. 363, 978-988, 2004). Disease-orientated fatigue is not caused by muscle overuse or physical impairment outside the central nervous system. This central fatigue is a subjective feeling with symptoms of disease-associated mental fatigue (a subjective feeling of having impaired concentration, reduced memory,
and speech difficulties) and disease-associated physical fatigue (subjective feeling of being exhausted and lacking energy). This subjective disease-associated fatigue can have its origin in particular embodiments by disturbance of the dopaminergic system of the CNS, particularly a lack of dopamine and potentially also homeostatic changes, which lead to an abnormal degree of persistent tiredness, weakness or exhaustion.
[0061] A well-accepted measurement of this type of fatigue is the FSS, which has been described above.
[0062] In particular embodiments, the score on the FSS is at least 4.
[0063] In particular embodiments, the score on the FSS is at least 4 for at least 2 weeks.
[0064] In particular embodiments, disease-associated fatigue is fatigue associated or caused by disturbance of the dopaminergic system, particularly a lack of dopamine.
[0065] As used herein, the term "subject" encompasses mammals including animals and humans.
[0066] The term "treat" is used herein to mean to relieve or alleviate at least one symptom of a disease in a subject. Within the meaning of the present invention, the term "treat" also denotes to arrest, delay the onset (i.e., the period prior to clinical manifestation of a disease) and/or reduce the risk of developing or worsening a disease. Thus, "treatment" as used herein includes modifying, curative and symptomatic treatments.
[0067] The term "therapeutically effective" applied to dose or amount refers to that quantity of a compound or pharmaceutical composition sufficient to result in a desired activity upon administration to a mammal in need thereof.
[0068] The phrase "pharmaceutically acceptable", as used in connection with compositions of the invention, refers to molecular entities and other ingredients of such compositions that are physiologically tolerable and do not typically produce untoward
reactions when administered to a mammal (e.g., human). The term "pharmaceutically acceptable" may also mean approved by a regulatory agency of the federal or a state government or listed in the U.S. Pharmacopeia or other generally recognized pharmacopeia for use in mammals, and more particularly in humans.
[0069] The term "salt" is defined as a chemical containing different charged components. The term salt also includes hydrates and solvates.
[0070] (R)-Phenylpiracetam may be used according to the invention in the form of any of pharmaceutically acceptable salts, solvates and conjugates. Any references to (R)- phenylpiracetam in this description should be understood as also referring to such salts, solvates and conjugates.
[0071] In certain embodiments, (R)-phenylpiracetam is present as (R)-phenylpiracetam hydrochloride.
[0072] In certain embodiments of the present invention, the disease-associated fatigue is a fatigue selected from the list of: fatigue in PD, fatigue in cancer, fatigue post-stroke, fatigue in burn-out syndrome, fatigue in multiple sclerosis, fatigue in HIV/AIDS and other infectious diseases of the CNS, fatigue in fibromyalgia, fatigue in sarkoidosis, fatigue in rheumatic disorders, fatigue in muscle dystrophies, fatigue in lupus erythematosus, fatigue in Morbus Crohn, fatigue in spondylitis ankylosans (Morbus Bechterew), fatigue in pulmonary arterial hypertension, fatigue in depression, fatigue in dementia, chronic fatigue syndrome, fatigue in chronic intoxication, fatigue in traumatic brain injury, fatigue in hypoxic brain damage, fatigue in hepatic infections, e.g. hepatitis C, fatigue in cirrhosis, e.g. primary biliary cirrhosis, fatigue in cachexia (age and tumor related), fatigue in amyotrophic lateral sclerosis (ALS), post-polio fatigue, and fatigue in myasthenia gravis.
[0073] In certain embodiments, the disease-associated fatigue is fatigue in Parkinson's disease.
[0074] In certain embodiments, the present invention relates to a method of treating fatigue-associated symptoms in PD, particularly inactivity, motivational-deficit, floppiness, exhaustion, lassitude, and prostration.
[0075] A well-accepted measurement of this type of fatigue is the Parkinson Fatigue Scale, which has been described above.
[0076] In certain embodiments, the disease-associated fatigue is mental fatigue in Parkinson's disease.
[0077] In certain embodiments, the score on the Parkinson Fatigue Scale is at least 7.
[0078] In certain embodiments, the score on the Parkinson Fatigue Scale is at least 7 for at least 2 weeks.
[0079] In certain embodiments, the disease-associated fatigue is fatigue in cancer.
[0080] In certain embodiments, the disease-associated fatigue is mental fatigue in cancer.
[0081] In certain embodiments, the present invention relates to a method of treating sleep-associated problems of PD patients, which have significant impact on their quality of life, particularly general tiredness, and drowsiness.
[0082] In certain embodiments of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 1 mg to about 400 mg/day.
[0083] In the context of the present invention, the term "about" or "approximately" means between 90% and 1 10% of a given value or range, i.e. "about 100" means "between 90 and 1 10". In narrower embodiments, the term "about" or "approximately" means between 95% and 105% of a given value or range, or between 98% and 102% of a given value or range, or between 99% and 101 % of a given value or range.
[0084] In a further embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 25 mg to about 350 mg/day.
[0085] In a still further embodiment, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from about 50 mg to about 300 mg/day, particularly in a range from about 50 mg to about 150 mg/day.
[0086] In yet another embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered once a day, particularly at a dose of about 200 mg once a day.
[0087] In yet another embodiment of the method of the present invention, (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a multiple dose, for example twice a day (bid.), or three times a day, particularly twice a day, particularly at a dose of about 100 mg twice a day.
[0088] In conjunction with the methods of the present invention, also provided are pharmaceutical compositions comprising a therapeutically effective amount of (R)- phenylpiracetam. The compositions of the invention may further comprise a carrier or excipient (all pharmaceutically acceptable). The compositions may be formulated e.g. for once-a-day administration, twice-a-day administration, or three times a day administration.
[0089] The term "carrier" applied to pharmaceutical compositions of the invention refers to a diluent, excipient, or vehicle with which an active compound (e.g., (R)- phenylpiracetam) is administered. Such pharmaceutical carriers may be sterile liquids, such as water, saline solutions, aqueous dextrose solutions, aqueous glycerol solutions, and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like. Suitable pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences" by A.R. Gennaro, 20th Edition.
[0090] The active ingredient (e.g. , (R)-phenylpiracetam) or the composition of the present invention may be used for the treatment of at least one of the mentioned disorders, wherein the treatment is adapted to or appropriately prepared for a specific administration as disclosed herein (e.g., to once-a-day, twice-a-day, or three times a day administration). For this purpose the package leaflet and/or the patient information contains corresponding information.
[0091] The active ingredient (e.g., (R)-phenylpiracetam) or the composition of the present invention may be used for the manufacture of a medicament for the treatment of at least one of the mentioned disorders, wherein the medicament is adapted to or appropriately prepared for a specific administration as disclosed herein (e.g., to once-a- day, twice-a-day, or three times a day administration). For this purpose the package leaflet and/or the patient information contains corresponding information.
[0092] According to the present invention, the dosage form of (R)-phenylpiracetam, or a (R)-phenylpiracetam salt, may be a solid, semisolid, or liquid formulation.
[0093] (R)-Phenylpiracetam may be administered via different application routes. The oral and the parenteral route are the preferred routes of application. (R)- Phenylpiracetam may be formulated as a flavored liquid, a capsule or a tablet.
[0094] For oral administration in the form of a tablet or capsule, (R)-phenylpiracetam may be combined with non-toxic, pharmaceutically acceptable excipients.
[0095] The optimal therapeutically effective amount may be determined experimentally, taking into consideration the exact mode of administration, form in which the drug is administered, the indication toward which the administration is directed, the subject involved (e.g., body weight, health, age, sex, etc.), and the preference and experience of the physician or veterinarian in charge.
[0096] Suitable daily doses of the active ingredient of the invention in therapeutic treatment of humans are within the range from about 1 mg to about 400 mg per day
(based on (R)-phenylpiracetam as free base), such as from about 25 mg to about 350 mg, or from about 50 mg to about 300 mg, particularly about 200 mg per day. In an alternative setting, the daily dose may be body weight-adjusted such as about 200 mg/day up to 80 kg body weight or 240 mg/day for patients with a body weight of≥ 80 kg. In a further alternative setting, (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is administered in a range from starting from about 50 mg and increasing the dose in 50 mg steps until the desired therapeutic efficacy is reached, but maximally to about 400 mg/day. Furthermore, in modified release formulations the total amount of active ingredient per day of administration could also be higher due to reduced bioavailability, e.g. up to about 500 mg/day. For use of a pharmaceutically acceptable salt, a solvate, a conjugate or a derivative of (R)-phenylpiracetam, such as (R)-phenylpiracetam hydrochloride, the corresponding amount may be adjusted so that an equimolar amount is used.
[0097] (R)-Phenylpiracetam may be administered as a single anti-fatigue agent or in combination with one or more additional pharmaceutical agents for the therapy of fatigue, particularly for the treatment of mental fatigue.
[0098] In particular such embodiments, said one or more additional pharmaceutical agents are selected from rasagiline and pramipexole.
[0099] In another embodiment, the pharmaceutical composition comprising (R)- phenylpiracetam may further comprise at least one additional active agent as defined herein, particularly wherein the additional active agent is for the treatment of the disease the fatigue is associated with. In particular such embodiments, the disease is Parkinson's disease (PD).
[00100] In an additional embodiment, (R)-phenylpiracetam is administered in combination with a drug, which increases the tolerability of the treatment with (R)- phenylpiracetam and/or reduces at least one side effect associated with the treatment with (R)-phenylpiracetam.
EXAMPLES
[00101] The following example illustrates the invention without limiting its scope.
EXAMPLE 1 : Determination of (R)-phenylpiracetam targets
[00102] Using functional monoamine transporter assays, we determined the potential drug targets for (R)-phenylpiracetam.
[00103] Recombinant Chinese hamster ovary (CHO) cells CHO-K1 (ATCC® CCL- 61™) cells stably expressing dopamine transporters are plated. The cells (2 x 105/ml) are pre-incubated with test compound and/or vehicle in modified Tris-HEPES buffer pH 7.1 at 25°C for 20 min and 50 nM [3H]-dopamine is then added for an additional 15 min incubation period. Non-specific signal is determined in the presence of 10 μΜ nomifensine. Cells are then solubilized with 1 % SDS lysis buffer. Reduction of [3H]- dopamine uptake by 50 percent or more (> 50%) relative to vehicle controls indicates significant inhibitory activity. Compounds are screened at 10, 1 , 0.1 , 0.01 and 0.001 μΜ. These same concentrations are concurrently applied to a separate group of untreated cells and evaluated for possible compound-induced cytotoxicity only if significant inhibition of uptake is observed.
[00104] Recombinant Madin Darby canine kidney (MDCK) cells (NBL-2) (ATCC® CCL-34™) expressing norepinephrine transporter are plated for two days. Test compound and/or vehicle is pre-incubated with cells (1 x 105/ml) in modified Tris- HEPES buffer pH 7.1 for 20 min at 25°C and 25 nM [3H]-norepinephrine is then added for an additional 15 min incubation period. A lysate is obtained from solubilized cells and counted to determine [3H]-norepinephrine uptake. Reduction of [3H]-norepinephrine uptake by 50 percent or more (> 50%) relative to 10 μΜ desipramine indicates significant inhibitory activity. Compounds are screened at 10, 1 , 0.1 , 0.01 and 0.001 μΜ. These same concentrations are concurrently applied to a separate group of untreated cells and evaluated for possible compound-induced cytotoxicity only if significant inhibition of uptake is observed.
[00105] These pharmacological experiments showed an affinity of (R)- phenylpiracetam for the neuronal dopamine re-uptake transporter of 13 μΜ in functional assays.
[00106] In microdialysis experiments, we could show that at a behaviorally active dose of 100 mg/kg a free concentration 50 μΜ is reached in extracellular fluid in the brain, and at 50 mg/kg a free concentration of 20 μΜ.
[00107] These concentrations are sufficiently high to postulate that the dopamine re-uptake transporter is a relevant target in the brain.
EXAMPLE 2: Assessment of (R)-phenylpiracetam on extracellular dopamine
levels in rat striatum using brain microdialysis - Fig. 1
Test Item
[00108] (R)-Phenylpiracetam was dissolved in saline and injected i.p.
Animals
[00109] Adult male Sprague-Dawley rats (n=5, -300 g; Harlan, The Netherlands) were used for the experiments. After surgery animals were housed individually (cages 30 cm x 30 cm x 40 cm) with food and water was ad libitum available at standard conditions. The post-surgery interval for recovery was minimally 48 h.
Surgery
[00110] Rats were anesthetized using isoflurane (2%, 800 ml/min O2) and placed in a stereotaxic frame (Kopf instruments, USA), l-shaped probes (Hospal AN 69 membrane, 3 mm exposed surface; Brainlink, the Netherlands) were inserted into striatum. Coordinates for the tips of the probes were: posterior (AP) = + 0.9 mm to bregma, lateral (L) = +3.0 mm to midline and ventral (V) = -6.5 mm to dura (Paxinos, G., and Watson, C. (1982) The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego).
Microdialysis experiments
[0011 ] Experiments were performed 24-48 hours after surgery. On the day of the experiment, the probes were connected with flexible polyether ether ketone (PEEK) tubing to a microperfusion pump (Syringe pump UV 8301501 , Univentor, Malta) and perfused with artificial cerebro-spinal fluid (aCSF), containing 147 mM NaCI, 3.0 mM KCI, 1.2 mM CaCI2, and 1 .2 mM MgCI2) at a flow rate of 1 .5 μΙ/min. Microdialysis samples were collected for 20 minute periods up to 120 min using automated fraction collector (Univentor 820 Microsampler, Antec, Netherlands), and stored at -80°C pending analysis. After the experiment, the rats were sacrificed and the brains were removed. The position of each probe was histologically verified according to Paxinos and Watson (loc. cit.) by making coronal sections of the brain.
Determination of Dopamine (DA) and 3,4-Dihydroxyphenylacetic acid (DOPAC) Separation of DA and DOPAC
[00112] Samples (20 μΙ) were injected onto the high-performance liquid chromatography (HPLC) column (Reversed Phase, particle size 3 μητι, C18, Thermo BDS Hypersil column, 150 x 2.1 mm, Thermo Scientific, USA) by a refrigerated microsampler system, consisting of a syringe pump (Gilson, model 402, France), a multi-column injector (Gilson, model 233 XL, France), and a temperature regulator (Gilson, model 832, France). Chromatographic separation was performed using a mobile phase that consisted of a sodium acetate buffer (6.15 g/l) with methyl alcohol (2.5% v/v), Titriplex (250 mg/l), 1-octanesulfonic acid (OSA, 150 mg/l), and adjusted with glacial acetic acid to pH 4.1 (isocratic). The mobile phase was run through the system at a flow rate of 0.35 ml/min by an HPLC pump (Shimadzu, model LC-10AD vp, Japan).
Detection
[001 13] Concentrations of DA and DOPAC were determined in the same sample, by HPLC separation and electrochemical detection. DA and DOPAC were detected electrochemically using a potentiostate (Antec Leyden, model Intro, the Netherlands) fitted with a glassy carbon electrode set at +500 mV vs. Ag/AgCI (Antec Leyden, the
Netherlands). Data was analyzed by Chromatography Data System (Shimadzu, class- p, Japan) software. Concentrations were quantified by the external standard method.
Statistical Analysis
[00114] After transmitter levels were stabilized, four consecutive pre-treatment microdialysis samples with less than 50% variation were taken as baseline and their mean was set at 100%. Transmitter concentration in each fraction was expressed as % ± SEM (standard error mean) of baseline.
Results
[00115] The results show that (R)-phenylpiracetam increases concentration of dopamine in the striatum.
EXAMPLE 3: Assessment of the concentration of (R)-phenylpiracetam in the
brain using brain microdialysis - Fig. 2
Subjects
[001 16] Naive adult male Sprague-Dawley rats (240-360 g, Janvier, France) were used for the study kept under standard conditions. All experiments were conducted during the light period of the day-night cycle.
Surgery
[001 17] Siliconized guide cannula (MAB 6.14.IC) (MAB, Stockholm, Sweden) were implanted unilaterally in pentobarbital anaesthetized animals aiming at the caudatus
putamen (CPu; AP: +0.1 , LM: ± 2.6, DV: -3.2 mm relative to bregma; -3.3 mm interaural) according to the atlas of Paxinos and Watson (loc. cit.). Rats were given at least 3 days to recover from surgery before starting microdialysis experiments.
Microdialysis
[001 18] Microdialysis experiments were performed in the home cage of the animal. A microdialysis probe (MAB 6.14.4.; 4 mm exposed membrane length, polyethersulfone (PES) membrane; MAB, Stockholm, Sweden) was lowered through the guide cannula into the CPu (ventral position of probe tip with reference to the skull: -7.2 mm) ca. 12 hours before the sampling and left in place for the whole testing period.
[001 19] The probes were perfused with aCSF at a flow rate of 2 μΙ/min using a CMA 102 perfusion pump (CMA, Solna, Sweden). The composition of the aCSF was 147 mM Na+, 2.7 mM K+, 1.2 mM Ca2+, 0.85 mM Mg2+, 0.04 mM ascorbic acid. The animals were connected by a head block tether system (Instech, Plymouth Meeting, USA) to a dual channel liquid swivel 375/D/22QM (Instech, Plymouth Meeting, USA). FEP tubing and tubing adapters (MAB, Stockholm, Sweden) were used. The sample collection began one hour after start of perfusion with three 20-minutes fractions (baseline). Thereafter, each rat was injected i.p. with (R)-phenylpiracetam and/or L- DOPA (25 mg/kg + benserazide, 15 mg/kg). The samples (40 pi) were collected automatically with a fraction collector (CMA/142; CMA, Solna, Sweden) and stored at - 20°C until analysis.
Determination of recovery
[00120] To perform in vitro recovery, probes were inserted into a beaker with aCSF (37°C) containing 100 nM solution of (R)-phenylpiracetam or L-DOPA. Five samples (40 μΙ) were collected while only the last 2 were used for analysis of (R)- phenylpiracetam or L-DOPA concentration.
Analytics
[00121 ] HPLC in combination with atmospheric pressure ionization tandem mass spectrometry (API-MS/ S) was employed (HPLC (Shimadzu Prominence, Duisburg, Germany) coupled to an API 4000 Q Trap (triple quadrupole, Applied Biosystems/MDS Sciex, Darmstadt, Germany) equipped with a Turbolonspray source (ESI)). The analytical column was a Onyx Monolithic C18 50 mm x 2 mm (Phenomenex, Aschaffenburg, Germany). The mobile phase consisted of the eluent A water and eluent B acetonitrile both containing 0.1 % of formic acid. The chromatographic run consisted of a gradient over 1.5 min from 5% acetonitrile in water at start until a mobile phase composition of 50% acetonitrile in water. A volume of 1 ml was injected in the API/MS/MS. A standard curve was used to calculate sample concentrations of studied agents in our samples.
Results
[00122] The results show that (R)-phenylpiracetam reaches brain levels sufficient to affect primary target (DA carrier).
EXAMPLE 4: Effect of (R)-phenylpiracetam on locomotor activity (horizontal
activity) in rats - Fig. 3
Animals
[00123] Experimentally naive adult male Sprague-Dawley rats (230-300 g) are kept 4 per cage, in a room with controlled temperature (21 ± 1°C) and humidity. Food and water are available ad libitum and the animals are kept under an alternating 12 h / 12 h day-night cycle (lights on at 07.00) for at least 6 days before the experiments are started. Each animal is used only once. Experimental group consist 8 animals per group.
Procedure
[00124] Locomotor activity was measured in 8 perspex boxes (ENV-515-16, 43.2 mm x 43.2 mm x 30 cm), Med-Associates Inc.) equipped with 4 arrays of 16 infrared photobeams placed 3 cm above the floor measure horizontal activity. Distance traveled (DT) was used in further analysis as a measure of locomotion.
Treatment
[00125] (R)-Phenylpiracetam was administered i.p.in a volume of 2 ml/kg in saline
Statistical analysis
[00126] Data with locomotor activity were analyzed by means of Kruskal-Wallis ANOVA on ranks followed if significant by rank sum test.
Results
[00127] Figure 3 shows that (R)-phenylpiracetam increases locomotor activity
(horizontal activity) in rats starting at the dose of 100 mg/kg indicating stimulatory activity.
EXAMPLE 5: Testing of (R)-phenylpiracetam in an EEG screen - Fig. 4
[00128] (R)-Phenylpiracetam was tested at four different concentrations (1 mg/kg, 12.5 mg/kg, 25 mg/kg, and 50 mg/kg) in an EEG screen as described by Dimpfel (Dimpfel, Neuropsychobiology, 58, 178-86, 2008).
Animals
[00129] Eight adult Fisher 344 rats (8 months of age, on inverse light-dark cycle, weight about 400 g, provided by Charles River Laboratories, D-97633, Sulzfeld) were used in this experimental series. Animals were implanted with electrodes into the brain and were given two weeks for recovery from surgery (for details see 6.4). After this the
transmitter was plugged in for adaptation and control experiments. During the recording rats were not restricted and could move freely but did not have food available (chewing would have produced too many artifacts).
Acclimatisation and housing conditions
[00130] The animals were allowed to acclimatize for at least 4 weeks before the study started. There was automatic control of light cycle, temperature and humidity. Light hours were 18h in the evening - 6h in the morning. Daily monitoring indicated that temperature and humidity remained within the target ranges of 22 ± 2°C and 44 ± 5% respectively. Cages, bedding, and water bottles were changed at regular intervals, i.e. every 2-3 days. Standard diet (Nohrlin H10, Altromin, D-32791 Lage, Germany) was^ available to the animals ad libitum. The animals had access to domestic quality mains water ad libitum.
Surgery
[00131] Rats were implanted with 4 bipolar concentric steel electrodes within a stereotactic surgical procedure during anaesthesia with ketamine. All four electrodes were placed 3 mm lateral within the left hemisphere. Dorsoventral coordinates were 4, 6, 4.2 and 8 mm and anterior coordinates were 3.7, 9.7, 5.7 and 12.2 mm for frontal cortex, striatum, hippocampus, and reticular formation, respectively (according to the atlas of Paxinos and Watson, 1982). A p re-constructed base plate carrying 4 bipolar stainless steel semi-micro electrodes (neurological electrodes "SNF 100" from Rhodes Medical Instruments, Inc., Summerland, CA 93067, USA) and a 5-pin-plug was fixed to the skull by dental cement interacting with 3 steel screws placed on distance into the bone. The distant recording spot of the electrode was the active electrode whereas the proximal spots of the four electrodes were connected to each other to give a reference. The base plate was carrying a plug to receive later on the transmitter (weight: 5.2 g including battery, 26 mm x 12 mm x 6 mm of size).
Experimental Procedure
[00132] Electroencephalographic (EEG) signals were recorded from frontal cortex, hippocampus, striatum and reticular formation of freely moving rats from inside a totally copper shielded room. Signals were wirelessly transmitted by a radio-telemetric system (Rhema Labortechnik, Hofheim, Germany, using 40 MHz as carrier frequency) and were amplified and processed as described earlier to give power spectra of 0.25 Hz resolution (Dimpfel et al. 1986; Dimpfel et al. 1988; Dimpfel et al. 1989; Dimpfel, 2003). In short, after automatic artifact rejection signals were collected in sweeps of 4 s duration and fast-fourier transformed using a Hanning window. Sampling frequency was 512 Hz. Four values were averaged to give a final sampling frequency of 128 Hz, well above the Nyquist frequency. The resulting electrical power spectra were divided into 6 specially defined frequency ranges (delta: 0.8 - 4.5 Hz; theta: 4.75 - 6.75 Hz; alphal : 7.00 - 9.50 Hz; alpha2: 9.75 - 12.50 Hz; betal : 12.75 - 18.50 Hz; beta2: 18.75 - 35.00 Hz). Spectra were averaged in steps of 3 minutes each and displayed on-line.
Test Items
[00133] (R)-Phenylpiracetam was injected i.p. in saline followed by recording of "Tele-Stereo-EEG" intracerebral field potentials in combination with a video tracking system for detection of changes in motility (GJB Datentechnik GmbH, D-98704 Langewiesen, Germany). This system recognized locomotion as well as stereotyped behaviour by following a contrast difference of the black transmitter on the head of the animal in comparison to its environment.
Treatment Groups
[00134] After surgery all animals were randomly allocated to treatment groups, such that the treatment groups were evenly distributed throughout the caging system. A crossover design with at least one week of drug holidays in between the administrations was used. After a pre-drug period of 45 min for pre-drug recording, drug effects were observed continuously on the screen (artifact control) for 300 min subdivided into 15 min periods after a lag time of 5 min for calming of animals after i.p. administration.
Changes of electrical power are expressed as % of the 45 min absolute pre-drug spectral power values within each frequency band.
Statistical Analysis
[00135] Data are expressed as mean values ± S.E.M. Statistics were calculated by means of the Wilcoxon-Mann-Whitney U-test for comparison to results obtained by vehicle injection at the particular time frame For comparison of data to reference compounds tested earlier under identical conditions discriminant analysis according to Fischer was used. A total of 24 variables (six frequency ranges times 4 brain areas) were used for analysis. Firstly, spherical projection of the results from 47 reference compounds plus physiological sleep was performed using the three spatial coordinates for the first three discriminant axes. Secondly, coding of the result of the fourth to sixth discriminant analysis into red, green and blue was followed by an additive color mixture in analogy to the so-called RGB mode (as used in TV). This matrix of drug actions is kept constant (frozen) for classification of unknown preparations since addition of further compounds would otherwise change the projections.
Results
[00136] (R)-Phenylpiracetam produced a dose dependent attenuation of alpha2 and betal waves (see Figure 4). The highest dosage produced a different pattern of changes in that theta power increases within the frontal cortex were observed. Motility was increased. Alpha2 waves are mainly under the control of dopamine (Dimpfel, loc. cit.). Thus, direct or indirect effects on dopaminergic neurotransmission can be expected from (R)-phenylpiracetam. Attenuation of alpha2 and betal waves has also been observed after administration drugs used for treatment of M. Parkinson (Dimpfel and Hoffmann, Neuropsychobiology, 62, 213-220, 2010). In summary (R)- phenylpiracetam shows a profile within the area of stimulatory drugs.
EXAMPLE 6: Effect of (R)-phenylpiracetam in motivation tests - Figs. 5, 6, 7, 8
Test Item
[00137] Methylphenidate purchased from Sigma (Taufkirchen, Germany) was dissolved in distilled water fresh for each test day. Modafinil purchased from Sequoia Research Products Limited (Pangbourne, UK) was dissolved in 1% w/v methylcellulose (Sigma, Taufkirchen, Germany) in 0.9% NaCI water fresh for each test day. (R)- phenylpiracetam was dissolved in distilled water fresh for each test day.
Animals
[00138] One hundred and three Male Sprague-Dawley rats (Janvier, Le Genest- St-lsle, France) weighing 225-250 g on arrival were used.
Acclimatisation and housing conditions
[00139] The animals were kept under standard laboratory conditions in groups of up to 4 per cage with ad libitum access to water. Standard diet for rodents (Altromin) was available to the animals at 15 g per animal/day.
Treatment Groups
[00140] Two experiments were performed using an identical protocol. After continuous reinforcement training (CRF) training (see below) all animals were assigned to treatment groups, such that performance across all the groups was similar. The treatment groups were evenly distributed throughout the caging system.
Experimental methods
[00141 ] In experiment 1 and 2 the following protocols were used:
[00142] Overview: Three tests were performed in a consecutive manner on subsequent days. On day 1-2, animals were habituated individually to Skinner boxes in two sessions, one per day, for 30 min. Animals received "free" food pellets at random time (one pellet per minute on average). On days 3-8 animals were trained in Skinner boxes on a continuous reinforcement schedule (one pellet per lever press). On days 9-
10 they were tested in the progressive ratio test (Test 1) off-drug, on days 1 1 -12 on- drug. On day 13, a choice test (Test 2) was performed on-drug, on day 14, a consumption test (Test 3) was performed on-drug. Below, tests are described in detail.
Test 1: Progressive ratio (PR) task
[00143] Behavioral testing was conducted in 12 operant test chambers (Med Associates, St. Albans, USA). Each chamber was equipped with a retractable lever, a food dispenser with receptacle, an overhead house light and two stimulus lights, one above the lever and the other above the food receptacle. An infrared photocell beam detected nose pokes into the food receptacle. The apparatus was controlled by a computer system (SmartControl®-lnterface and Med PC-software, Med Associates, St. Albans, USA). The light above the food receptacle indicated the delivery of a food pellet in the receptacle. Rats were first habituated to the operant boxes for two sessions (30 min each) on two consecutive days. Thereafter, animals were trained for six sessions on the CRF schedule for 30 min. Followed by 4 sessions (two session off-drug followed by two sessions on-drug) of an increasing fixed ratio (FR) schedule in steps of 5, with 3 repetitions of each step (i.e. 1-1-1 ; 5-5-5; 10-10-10; ...). When the required FR value of each trial was achieved the light above the food receptacle indicated the delivery of a single food pellet (45 mg, Bioserve, USA). The light remained on until the rat poked its nose in the receptacle. Lever presses while the light was on were counted as perseverative lever presses; they were counted but had no programmed consequences. A session lasted for 90 min or was ended when a rat failed to press the lever for consecutive 10 min. For each session, the value of the last completed ratio (breaking point) was recorded as well as the amount of received rewards, the perseverative lever presses, the duration of the session and the latency to respond. Animals of all treatment groups received respective vehicle/drug infusions 30 min pre-test on the final two sessions involving testing under a PR schedule.
Test 2: Cost-benefit choice test
[00144] In this task the rats had the choice between working for their preferred food (Bioserve pellets) by pressing the lever on a PR schedule as described above or
obtaining lab chow being freely available in a dish (about 15 g) within the operant chamber. The food receptacle and the lever were positioned on the same wall of the operant chamber, the dish containing the lab chow was situated in a corner on the opposite side of the operant chamber. A session lasted for 90 min or ended when a rat failed to press the lever for consecutive 10 min. Thereafter the amount of lab chow ingested was calculated. Animals of all treatment groups received vehicle/drug infusions 30 min pre-test.
Test 3: Consumption test
[00145] The animals were placed individually in separate cages (type III) containing a glass bowls filled with 30 g of pellets. The animals had free access to the reward for 20 min and the amount consumed was measured by subtracting the weight of each glass bowl before the 20 min consumption test and the weight of each glass bowl after the test. Animals of all treatment groups received vehicle/drug infusions 30 min pre-test.
Statistical Analysis
[00146] The data were subjected to one or two way ANOVAs followed by Dunnett's post hoc test. All statistical computations were carried out with STATISTICA™ (StatSoft®, Tulsa, USA). The level of statistical significance (a-level) was set at p < 0.05.
Results
[00147] Present data show that (R)-phenylpiracetam increases motivation, i.e., the work load, which the animals are willing to perform to obtain more rewarding food. At the same time consumption of freely available normal food does not increase. Generally this indicates that (R)-phenylpiracetam increases motivation and in turn effect on mental fatigue would be expected. The effect of (R)-phenylpiracetam is much stronger than that of methylphenidate and amphetamine. Moreover, (S)-phenylpiracetam produces only a very weak effect at 100 mg/kg, which is 2.5 times lower than (R)-phenylpiracetam, and no significant effect at 200 mg/kg.
EXAMPLE 7: Effect of (R)-phenylpiracetam on rats with unilateral SNc lesion
(model of Parkinson s Disease) - Fig. 9
[00148] (R)-Phenylpiracetam was tested in rats with unilateral SNc lesions - a preclinical model of Parkinson's disease. In this model, ipsilateral rotations indicate a presynaptic mode of action, which is consistent with inhibition of dopamine uptake as a primary mode of action.
Animals
[00149] Male Sprague-Dawley rats that have undergone unilateral lesion of medial forebrain bundle using 6-hydroxydopamine and showed a clear-cut ipsilateral rotation bias in amphetamine rotation test. The rats were housed four per cage in the animal room with a controlled 12-hour light-dark cycle and controlled temperature (21 °C) with access to standard laboratory food (chow pellets) and tap water ad libitum. All experiments were carried out between 09:00 and 16:00.
Chemicals
[00150] (R)-Phenylpiracetam was injected i.p. in saline.
Rotation test
[00151] Rats were injected with substance, placed in Perspex cylinders (30 cm diameter), and the rotational behaviour (360°) was scored up to 360 min at 20-min intervals using TSA rotation measurement system.
Statistical analysis
[00152] Data are presented as means +/- SEM. Sums of total scores obtained during a whole recorded period was analysed using two-way ANOVA. If significant, the two-way ANOVA was followed by the Dunnett test for pair-wise multiple comparisons.
Results
[00 53] This study showed that (R)-phenylpiracetam increased ipsilateral rotations in a PD animal model. These results suggest that (R)-phenylpiracetam improves fatigue associated with Parkinson's disease. In summary, these results suggest that (R)- phenylpiracetam that fulfils the criteria of an effective and well-tolerated treatment for PD patients suffering from fatigue and fatigue-associated symptoms, like inactivity, motivational-deficit, apathy, floppiness, exhaustion, lassitude, prostration etc.
[00154] Based on these initial findings, we can conclude that (R)-phenylpiracetam is a candidate for the treatment of fatigue associated with PD due to its effect on the dopamine re-uptake transporter.
EXAMPLE 8: Effect of (R)-phenylpiracetam on sedation induced by reserpine -
Fig. 10
Animals
[00155] Male Sprague-Dawley rats weighing ca. 300 g were kept under standard laboratory conditions.
Chemicals
[00156] Animals were injected with reserpine (5 mg/kg) and a-MT (250 mg/kg) 24 and 3.5 h before testing, respectively. (R)-phenylpiracetam (25, 50 or 100 mg/kg) was injected i.p. directly before the test.
Locomotor activity in reserpine-treated rats
[00157] The locomotor activity was measured in four perspex boxes (ENV-515-16, 43.2 cm x 43.2 cm x 30 cm), Med-Associates Inc. system) equipped with 4 arrays of 16 infrared photobeams placed 3 cm above the box floor. Distance travelled (DT) was used
in further analysis for measuring locomotion. The recording started immediately after placing animals in the open field.
Statistical analysis
[00158] Sums of total scores obtained during a whole recorded period was analysed by one-way ANOVA.
Results
[00159] The data show that (R)-phenylpiracetam attenuates sedation produced by reserpine supporting anti-fatigue activity.
EXAMPLE 9: Effect of (R)-phenylpiracetam on sedation induced by haloperidol - Fifl- 11
Animals
[00160] Male Sprague-Dawley rats weighing ca. 300 g were kept under standard laboratory conditions.
Chemicals
[00161] (R)-Phenylpiracetam (25, 50 and 100 mg/kg) was injected i.p. in saline directly before the test. Haloperidol was also given i.p. at the dose of 0.2 mg/kg, 30 min before the test start.
Locomotor activity in haloperidol-treated rats
[00162] The locomotor activity was measured in four perspex boxes (ENV-515-16, 43.2 cm x 43.2 cm x 30 cm), Med-Associates Inc. system) equipped with 4 arrays of 16 infrared photobeams placed 3 cm above the box floor. Distance travelled (DT) was used
in further analysis for measuring locomotion. The recording started immediately after placing animals in the open field.
Statistical analysis
[00163] Sums of total scores obtained during a whole recorded period was analysed by one-way ANOVA followed, if significant, by the Holm-Sidak test.
Results
[00164] The data show that (R)-phenylpiracetam dose-dependently attenuates sedation produced by haloperidol supporting anti-fatigue activity (Fig. 1 1 ). An additional experiment shows that (S)-phenylpiracetam is much less potent, producing no effect at 50 mg/kg (Fig. 12A) or an almost negligible effect at 100 mg/kg (Fig. 12B). It should be noted that (R)-phenylpiracetam produced a very robust effect at these doses.
References
Dimpfel, W. (2003). "Preclinical data base of pharmaco-specific rat EEG fingerprints
(tele-stereo-EEG)." Eur J Med Res 8(5): 199-207.
Dimpfel, W., M. Spuler, et al. (1988). "Monitoring of the effects of antidepressant drugs in the freely moving rat by radioelectroencephalography (tele-stereo-EEG)."
Neuropsychobiology 19(2): 116-120.
Dimpfel, W., M. Spuler, et al. (1989). "Hallucinogenic and stimulatory amphetamine derivatives: fingerprinting DOM, DOI, DOB, MDMA, and MBDB by spectral analysis of brain field potentials in the freely moving rat (Tele-Stereo-EEG)."
Psychopharmacology (Berl) 98(3): 297-303.
Dimpfel, W., M. Spuler, et al. (1986). "Radioelectroencephalography (Tele-Stereo-EEG) in the rat as a pharmacological model to differentiate the central action of flupirtine from that of opiates, diazepam and phenobarbital." Neuropsychobiology 16(2-3):
163-168.
Krosser, S. and J. Tillner (2007). "Pharmacokinetics of sarizotan after oral administration of single and repeated doses in healthy subjects." International Journal of Clinical Pharmacology and Therapeutics 45: 271-280.
[00165] The present invention is not to be limited in scope by the specific embodiments described herein. Indeed, various modifications of the invention in addition to those described herein will become apparent to those skilled in the art from the foregoing description. Such modifications are intended to fall within the scope of the appended claims.
[00166] To the extent possible under the respective patent law, all patents, applications, publications, test methods, literature, and other materials cited herein are hereby incorporated by reference.
Claims
1. A pharmaceutical composition comprising (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof for use in the treatment of disease- associated fatigue, particularly disease-associated mental fatigue.
2. The pharmaceutical composition according to claim 1 , wherein (R)-phenylpiracetam is present as (R)-phenylpiracetam hydrochloride.
3. The pharmaceutical composition according to claim 1 or 2, wherein the score on the Fatigue Severity Scale is at least 4.
4. The pharmaceutical composition according to claim 3, wherein the score on the Fatigue Severity Scale is at least 4 for at least 2 weeks.
5. The pharmaceutical composition according to any one of claims 1 to 4, wherein said disease-associated fatigue is fatigue associated with Parkinson's disease.
6. The pharmaceutical composition according to claim 5, wherein said disease- associated fatigue is mental fatigue associated with Parkinson's disease.
7. The pharmaceutical composition according to claim 5 or 6, wherein the score on the Parkinson Fatigue Scale is at least 7.
8. The pharmaceutical composition according to claim 7, wherein the Parkinson Fatigue Scale is at least 7 for at least 2 weeks.
9. The pharmaceutical composition according to any one of claims 1 to 8, wherein (R)- phenylpiracetam is for administration in a range from about 1 mg to about 400 mg/day, or in a range from about 25 mg to about 350 mg/day, or in a range from about 50 mg to about 300 mg/day.
10. The pharmaceutical composition according to any one of the preceding claims, wherein (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration once a day, twice a day, or three times a day.
11. The pharmaceutical composition according to any one of the preceding claims, wherein (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in an oral formulation.
12. The pharmaceutical composition according to any one of the preceding claims, wherein (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in combination with at least one additional pharmaceutical agent which has been shown to be effective for the treatment of disease-associated fatigue.
13. The pharmaceutical composition according to claim 12, wherein said disease- associated fatigue is fatigue associated with Parkinson's disease.
14. The pharmaceutical composition according to claim 13, wherein said disease- associated fatigue is mental fatigue associated with Parkinson's disease
15. The pharmaceutical composition according to claim 13 or 14, wherein said at least one additional pharmaceutical agent is selected from: rasagiline and pramipexole. 6. A method of treating disease-associated fatigue, particularly disease-associated mental fatigue, in a subject in need thereof, comprising the step of administering an effective amount of (R)-phenylpiracetam or a pharmaceutically acceptable salt thereof.
17. The method according to claim 16, wherein (R)-phenylpiracetam is present as (R)- phenylpiracetam hydrochloride.
18. The method according to claim 16 or 17, wherein the score on the Fatigue Severity Scale is at least 4.
19. The method according to claim 18, wherein the score on the Fatigue Severity Scale is at least 4 for at least 2 weeks.
20. The method according to any one of claims 16 to 19, wherein said disease- associated fatigue is fatigue associated with Parkinson's disease.
21 . The method according to claim 20, wherein said disease-associated fatigue is mental fatigue associated with Parkinson's disease.
22. The method according to claim 20 or 21 , wherein the score on the Parkinson Fatigue Scale is at least 7.
23. The method according to claim 22, wherein the Parkinson Fatigue Scale is at least 7 for at least 2 weeks.
24. The method according to any one of claims 16 to 23, wherein (R)-phenylpiracetam is for administration in a range from about 1 mg to about 400 mg/day, or in a range from about 25 mg to about 350 mg/day, or in a range from about 50 mg to about 300 mg/day.
25. The method according to any one of the preceding claims, wherein (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration once a day, twice a day, or three times a day.
26. The method according to any one of the preceding claims, wherein (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in an oral formulation.
27. The method according to any one of the preceding claims, wherein (R)- phenylpiracetam or a pharmaceutically acceptable salt thereof is for administration in combination with at least one additional pharmaceutical agent which has been shown to be effective for the treatment of disease-associated fatigue.
28. The method according to claim 27, wherein said disease-associated fatigue is fatigue associated with Parkinson's disease.
29. The method according to claim 28, wherein said disease-associated fatigue is mental fatigue associated with Parkinson's disease
30. The method according to claim 28 or 29, wherein said at least one additional pharmaceutical agent is selected from: rasagiline and pramipexole.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261668063P | 2012-07-05 | 2012-07-05 | |
| US61/668,063 | 2012-07-05 | ||
| EP12004987.9 | 2012-07-05 | ||
| EP12004987 | 2012-07-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014005720A1 true WO2014005720A1 (en) | 2014-01-09 |
Family
ID=49881369
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2013/001990 WO2014005720A1 (en) | 2012-07-05 | 2013-07-05 | Use of (r)-phenylpiracetam for the treatment of disease-associated fatigue |
Country Status (2)
| Country | Link |
|---|---|
| TW (1) | TW201408293A (en) |
| WO (1) | WO2014005720A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2891491A1 (en) * | 2014-01-03 | 2015-07-08 | Merz Pharma GmbH & Co. KGaA | Use of (r)-phenylpiracetam for the treatment of sleep disorders |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001062726A2 (en) * | 2000-02-23 | 2001-08-30 | Ucb, S.A. | 2-oxo-1-pyrrolidine derivatives, processes for preparing them and their uses |
| WO2007104780A2 (en) | 2006-03-16 | 2007-09-20 | Akciju Sabiedriba 'olainfarm' | N- carbamoylmethyl- 4- (r) -phenyl-2-pyrr0lidin0ne, method of its preparation and pharmaceutical use |
| EP2011497A1 (en) * | 2006-03-28 | 2009-01-07 | Akhapkina, Valentina Ivanova | Agent exhibiting a neurotropic, neuromodulator, cerebrovascular and anti- stroke activity |
-
2013
- 2013-07-03 TW TW102123814A patent/TW201408293A/en unknown
- 2013-07-05 WO PCT/EP2013/001990 patent/WO2014005720A1/en active Application Filing
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001062726A2 (en) * | 2000-02-23 | 2001-08-30 | Ucb, S.A. | 2-oxo-1-pyrrolidine derivatives, processes for preparing them and their uses |
| WO2007104780A2 (en) | 2006-03-16 | 2007-09-20 | Akciju Sabiedriba 'olainfarm' | N- carbamoylmethyl- 4- (r) -phenyl-2-pyrr0lidin0ne, method of its preparation and pharmaceutical use |
| EP2011497A1 (en) * | 2006-03-28 | 2009-01-07 | Akhapkina, Valentina Ivanova | Agent exhibiting a neurotropic, neuromodulator, cerebrovascular and anti- stroke activity |
Non-Patent Citations (36)
| Title |
|---|
| AKHAPKINA ET AL., ATMOSFERA. NERVNYE BOLEZNI, vol. 3, 2004, pages 28 - 31 |
| BEISKE ET AL., MOV DISORD., vol. 25, no. 14, 30 October 2010 (2010-10-30), pages 2456 - 60 |
| BEISKE; SVENSSON, ACTA NEUROL SCAND SUPPL., 2010, pages 78 - 81 |
| BEL'SKAIA ET AL., ZH NEVROL PSIKHIATR 1M S S KORSAKOVA, vol. 107, 2007, pages 40 - 43 |
| BOBKOV ET AL., BIULL EKSP BIOL MED., vol. 95, 1983, pages 50 - 53 |
| BROWN RG ET AL., PARKINSONISM RELATED DISORDERS, vol. 11, no. 1, 2005, pages 49 - 55 |
| CHAUDHURI; BEHAN, J NEUROL SCI., vol. 179, no. 1-2, 2000, pages 34 - 42 |
| CHAUDHURI; BEHAN, LANCET, vol. 363, 2004, pages 978 - 988 |
| DIMPFEL, NEUROPSYCHOBIOLOGY, vol. 58, 2008, pages 178 - 86 |
| DIMPFEL, W.: "Preclinical data base of pharmaco-specific rat EEG fingerprints (tele-stereo-EEG).", EUR J MED RES, vol. 8, no. 5, 2003, pages 199 - 207 |
| DIMPFEL, W.; M. SPULER ET AL.: "Hallucinogenic and stimulatory amphetamine derivatives: fingerprinting DOM, DOI, DOB, MDMA, and MBDB by spectral analysis of brain field potentials in the freely moving rat (Tele-Stereo-EEG).", PSYCHOPHARMACOLOGY (BERL, vol. 98, no. 3, 1989, pages 297 - 303 |
| DIMPFEL, W.; M. SPULER ET AL.: "Monitoring of the effects of antidepressant drugs in the freely moving rat by radioelectroencephalography (tele-stereo-EEG).", NEUROPSYCHOBIOLOGY, vol. 19, no. 2, 1988, pages 116 - 120 |
| DIMPFEL, W.; M. SPULER ET AL.: "Radioelectroencephalography (Tele-Stereo-EEG) in the rat as a pharmacological model to differentiate the central action of flupirtine from that of opiates, diazepam and phenobarbital.", NEUROPSYCHOBIOLOGY, vol. 16, no. 2-3, 1986, pages 163 - 168 |
| DIMPFEL; HOFFMANN, NEUROPSYCHOBIOLOGY, vol. 62, 2010, pages 213 - 220 |
| FRIEDMAN JH ET AL., MOV. DISORDERS, vol. 25, 2010, pages 805 - 822 |
| GALLAGHER ET AL., MOV DISORD., vol. 25, no. 15, 15 November 2010 (2010-11-15), pages 2493 - 500 |
| GERASIMOVA ET AL., ZH NEVROL PSIKHIATR 1M S S KORSAKOVA, vol. 105, 2005, pages 63 - 64 |
| GUSTOV ET AL., ZH NEVROL PSIKHIATR IM S S KORSAKOVA, vol. 106, 2006, pages 52 - 53 |
| HAVLIKOVA ET AL., EUR J NEUROL., vol. 15, no. 5, 2008, pages 475 - 80 |
| HAVLIKOVA ET AL., J NEUROL SCI., vol. 270, 2008, pages 107 - 13 |
| HAVLIKOVA ET AL., PARKINSONISM RELAT DISORD., vol. 14, 2008, pages 187 - 92 |
| KALINSKIJ; NAZAROV, ZH NEVROL PSIKHIATR 1M SS KORSAKOVA, vol. 107, 2007, pages 61 - 63 |
| KALINSKIJ; NAZAROV, ZH NEVROL PSIKHIATR IM S S KORSAKOVA, vol. 107, 2007, pages 61 - 63 |
| KARABANOV ET AL., ATMOSFERA. NERVNYE BOLEZNI, vol. 4, 2009, pages 29 - 32 |
| KR6SSER, S.; J. TILLNER: "Pharmacokinetics of sarizotan after oral administration of single and repeated doses in healthy subjects.", INTERNATIONAL JOURNAL OF CLINICAL PHARMACOLOGY AND THERAPEUTICS, vol. 45, 2007, pages 271 - 280 |
| KRUPP ET AL., ARCH NEUROL., vol. 46, 1989, pages 1121 - 23 |
| LYBZIKOVA ET AL., ZH NEVROL PSIKHIATR 1M S S KORSAKOVA., vol. 108, 2008, pages 69 - 70 |
| MALYKH ANDREI G ET AL: "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders", DRUGS,, vol. 70, no. 3, 1 January 2010 (2010-01-01), pages 287 - 312, XP009162620, ISSN: 0012-6667 * |
| MENDONCA ET AL., MOV. DISORD., vol. 22, 2007, pages 2070 - 2076 |
| MORITA ET AL., INTERN MED., vol. 50, 2011, pages 2163 - 2168 |
| NOVIKOVA ET AL., STOMATOLOGIIA (MOSK, vol. 87, 2008, pages 41 - 45 |
| PAXINOS, G.; WATSON, C.: "The Rat Brain in Stereotaxic Coordinates", 1982, ACADEMIC PRESS |
| RASCOL ET AL., LANCET NEUROL., vol. 10, 2011, pages 415 - 423 |
| SAVCHENKO ET AL., ZH NEVROL PSIKHIATR 1M S S KORSAKOVA., vol. 105, 2005, pages 22 - 26 |
| TIURENKOV ET AL., EKSP KLIN FARMAKOL., vol. 70, 2007, pages 24 - 29 |
| ZVEJNIECE ET AL., BASIC CLIN PHARMACOL TOXICOL., vol. 109, 2011, pages 407 - 12 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2891491A1 (en) * | 2014-01-03 | 2015-07-08 | Merz Pharma GmbH & Co. KGaA | Use of (r)-phenylpiracetam for the treatment of sleep disorders |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201408293A (en) | 2014-03-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101754045B1 (en) | Use of flecainide as an anti-connexin agent and method for potentiating the effects of a psychotropic drug | |
| KR20100014392A (en) | Droxidopa and pharmaceutical composition thereof for the treatment of fibromyalgia | |
| CA2766282C (en) | Inhibitors of carnitin-palmitoyl-transferase-1 for the treatment and prevention of disorders caused by delipidation of neural tissue | |
| TW201505634A (en) | Method for non-toxic treatment for drug withdrawal | |
| AU2020204420A1 (en) | Dosage forms and therapeutic uses of l-4-chlorokynurenine | |
| IL168853A (en) | Use of barbituric acid derivatives in the preparation of a medicament for treating movement disorders | |
| CN113473983A (en) | Methods of treating parkinson's disease by administration of resiniferatoxin | |
| US20060189612A1 (en) | Pharmaceutically active morpholinol | |
| KR102232198B1 (en) | (r)-pirlindole and its pharmaceutically acceptable salts for use in medicine | |
| JP2017523982A (en) | A pharmaceutical composition for preventing, treating, or delaying Alzheimer's disease or dementia, comprising a GPCR19 agonist as an active ingredient | |
| WO2014005721A1 (en) | Use of (r)-phenylpiracetam for the treatment of parkinson's disease | |
| TWI659017B (en) | Use of indolyl and indolinyl hydroxamates for treating neurodegenerative disorders or cognitive deficits | |
| EP3409275B1 (en) | Composition for prevention and/or treatment of diseases associated to hyperalgesia | |
| CN114007607A (en) | Materials and methods for treating neurodegenerative diseases | |
| KR102635941B1 (en) | Use of carbamate compounds for the prevention, alleviation or treatment of tremor or tremor syndrome | |
| WO2014005720A1 (en) | Use of (r)-phenylpiracetam for the treatment of disease-associated fatigue | |
| US11660279B2 (en) | Therapeutic agents for treating restless leg syndrome | |
| TW202308653A (en) | Methods of treatment with neuroactive steroids | |
| JP2021500342A (en) | Drugs for neurodegenerative diseases | |
| RU2802288C2 (en) | Therapeutic agents for the treatment of restless leg syndrome | |
| JP4427329B2 (en) | 2-Indanylamino derivatives for the treatment of chronic pain | |
| EP2891491A1 (en) | Use of (r)-phenylpiracetam for the treatment of sleep disorders | |
| US20180177748A1 (en) | Combination medication for neuro-degenerative diseases | |
| US20240058326A1 (en) | Methods of treating mental or mood disorders using 2-bromo-lsd | |
| CN117503756A (en) | Application of R-carvedilol in preparation of antidepressant drugs |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13737799 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13737799 Country of ref document: EP Kind code of ref document: A1 |