Abstract
Wheat and barley rank among the main crops cultivated on a global scale, providing the essential nutritional foundation for both humans and animals. Nevertheless, these crops are vulnerable to several fungal diseases, such as Septoria tritici blotch and net blotch, which significantly reduce yields by adversely affecting leaves and grain quality. To mitigate the effect of these diseases, chemical fungicides have proven to be genuinely effective; however, they impose a serious environmental burden. Currently, biocontrol agents have attracted attention as a sustainable alternative to fungicides, offering an eco-friendly option. The study aimed to assess the efficacy of Bacillus velezensis BE2 in reducing disease symptoms caused by Zymoseptoria tritici and Pyrenophora teres. This bacterium exhibited significant antagonistic effects in vitro by suppressing fungal development when pathogens and the beneficial strain were in direct confrontation. These findings were subsequently confirmed through microscopic analysis, which illustrated the strain’s capacity to inhibit spore germination and mycelial growth in both pathogens. Additionally, the study analysed the cell-free supernatant of the bacterium using UPLC-MS (ultra-performance liquid chromatography-mass spectrometry). The results revealed that strain BE2 produces, among other metabolites, different families of cyclic lipopeptides that may be involved in biocontrol. Furthermore, the beneficial effects of strain BE2 in planta were assessed by quantifying the fungal DNA content directly at the leaf level after bacterization, using two different application methods (foliar and drenching). The results indicated that applying the beneficial bacterium at the root level significantly reduced pathogens pressure. Finally, gene expression analysis of different markers showed that BE2 application induced a priming effect within the first hours after infection.
Key points
• BE2 managed Z. tritici and P. teres by direct antagonism and induced systemic resistance.
• Strain BE2 produced seven metabolite families, including three cyclic lipopeptides.
• Application of strain BE2 at the root level triggered plant defense mechanisms.
Similar content being viewed by others
Introduction
Ensuring the stability of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) production is of utmost importance, given that they are the primary staple food for approximately one-third of the world’s population (Watson et al. 2018). However, the production of these crops is negatively affected by biotic and abiotic stresses, resulting in direct adverse effects on yield and substantial economic losses. Among the biotic stresses, phytopathogenic fungi pose a particularly serious threat by attacking different plant organs. Septoria tritici blotch (STB) is a highly devasting diseases in wheat, causing yield losses of up to 50%. The causative agent of STB is the fungus Zymoseptoria tritici (in its anamorph or asexual form), predominantly found in Europe and known to infects wheat leaves when environmental conditions are favourable (Brokenshire 2007; Shewry 2009). This fungus is distinguished as hemibiotrophic, characterized by an extended biotrophic phase followed by a transition to the necrotrophic phase. During this transition, Z. tritici produces cell-wall-degrading enzymes that facilitate nutrients release from mesophyll cells, essential for sporulation (Lovell et al. 2004; Selim et al. 2014; Palma-Guerrero et al. 2017; Platel et al. 2021).
In the case of barley, net blotch is a severe disease that poses a significant threat to the brewing sector. The causal pathogen of net blotch is Pyrenophora teres, which can be further classified into two different forms: P. teres f. teres and P. teres f. maculata, distinguished by the type of the lesions they induce on barley leaves. Similar to Z. tritici, P. teres is also a hemibiotrophic pathogen, but it exhibits shorter biotrophic and incubation periods (Liu et al. 2011; Backes et al. 2021b).
Due to the varying modes of action exhibited by these pathogens, farmers must adapt their control strategies accordingly. Even though the resistant crop varieties can decrease the impact of fungi, they are not fully effective, requiring additional measures like chemical products to reduce yield losses (Morin 2020). However, pesticide use leads to various health and environmental issues, including pathogen resistance (Rupp et al. 2017). Thus, there is a pressing necessity for an alternative strategy employing plant-associated bacteria or their secondary metabolites to control plant diseases and enhance crop yields.
The Bacillus genus includes different species that inhabit different ecological niches such as soils, air, water, plants, and animals (Elshaghabee et al. 2017). They are Gram-positive and have a rod-shaped structure. Among soil bacteria, Bacillus members are notably abundant, particularly in plant rhizosphere (Hardoim et al. 2015). Many studies have demonstrated the presence of Bacillus members with various plants, including date palm, wheat, barley, and rice (El Arbi et al. 2016; Rekha et al. 2018; Albdaiwi et al. 2019).
Different Bacillus strains were shown to have a positive effect on the plant growth development of many species. They enhance plant health and growth through the production of active molecules like indole-3-acetic acid (IAA) and 1-aminocyclopropane-1-carboxylate (ACC), which influence plant productivity. They also improve micronutrients availability by producing siderophore and phosphate solubilization (Sabir et al. 2012; Albdaiwi et al. 2019).
Bacillus strains act against many pathogens via a number of mechanisms, such as competing for resources, stimulating plant defenses, and producing compounds like enzymes, volatile compounds, and secondary metabolites (Compant et al. 2005; Anckaert et al. 2021; Lahlali et al. 2022). The genus Bacillus is a valuable source of a wide spectrum of secondary metabolites, especially of those produced by non-ribosomal enzymatic complexes responsible for the biosynthesis of the so-called non-ribosomal peptides (NRPs) and polyketides (PKs); both of which showing a great potential application in biocontrol (Leclère et al. 2005; Ongena and Jacques 2008; Esmaeel et al. 2018; Dutilloy et al. 2022).
Plant-pathogen interactions can lead to compatible or incompatible interaction, which results in transcriptomic and metabolomic modifications (Backes et al. 2021a). The first modifications following pathogen perception typically include alterations in the plant cell wall and the accumulation of reactive oxygen species (ROS) (Torres et al. 2006). ROS production plays a key role depending on the pathogen’s lifestyle. Biotrophic pathogens are known to be inhibited by the production of ROS, whereas the oxidative burst appears to benefit necrotrophic ones. Modulation of ROS and catalases is crucial during plant-hemibiotrophic pathogens, as demonstrated in cases involving Z. tritici and P. teres (Able 2003; Shetty et al. 2007; Lightfoot et al. 2017). Additionally, the regulation of phytohormones like ethylene, jasmonic acid (JA), and salicylic acid (SA) is essential for inducing systemic acquired resistance (SAR) and induced systemic resistance (ISR). Activation of these pathways triggers the expression of pathogenesis-related (PR) genes, especially PR1, PR2, and PR5 (Villena et al. 2018).
In the current study, the biocontrol activity of Bacillus velezensis BE2 (DSM 115787), isolated from the maize rhizosphere, against Z. tritici and P. teres was investigated. The efficacy of the bacterium was also assessed directly in planta by evaluating two application modes. This approach has resulted in a deeper understanding of the mechanisms involved in the tripartite interaction between the plant, the beneficial bacterium, and the pathogens, with particularly focus on the activation of plant defense genes. Furthermore, the complete genome sequence of strain BE2 was thoroughly examined to decipher its potential involvement in secondary metabolites production, guided by the presence of synthetic gene clusters. Additionally, specific metabolites produced by strain BE2 were identified through the use of ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) and UPLC-MS/MS analyses.
Materials and methods
Primers, strains, and culture condition
The BE2 strain was isolated from the maize rhizosphere in the northeastern France, following the protocol previously described by Esmaeel et al. (2020). The bacterium identity was established through a combination of phenotypic and biochemical characterization, encompassing aspects like morphology, size, mobility, growth on the API 50CHB/E medium. Additionally, the strain’s identity was confirmed by conducting 16S rRNA amplification using FD2 forward (5′ AGAGTTTGATCATGGCTCAG) and PR1 reverse (5′ ACGGTTACCTTGTTACGACTT) primers. The BE2 strain has been deposited in the publicly accessible DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), under the accession number DSM 115797.
The BE2 strain was cultivated on Luria Bertani (LB) medium (per liter: tryptone 10 g, yeast extract 5 g, NaCl 5 g, pH 7.2). For long-term storage, cells were mixed with LB broth containing 20% glycerol and then stored at − 80 °C. For metabolite production, the strain was grown in Landy medium (per liter: 1 g/L yeast extract, 0.5 g/L MgSO4, 1 g/L K2HPO4, 0.5 g/L KCl, 0.0016 g/L CuSO4, 0.0024 g/L FeSO4, 0.0008 g/L NaSO4, 20 g/L glucose, 5 g/L glutamic acid, 21 g/L MOPS, 0.016 g/L L-tryptophane, dH2O to 100 mL) (Landy et al. 1948).
The pathogenic barley strain, P. teres MUCL 28818, was maintained on potato dextrose agar (PDA) medium (per liter: dextrose 20 g; potato extract 4 g and agar 17 g) at 20 °C for two weeks. To induce sporulation, one plug from the original culture was transferred onto V8 juice PDA medium (per liter: agar 10 g, CaCO3 3 g, 150 mL of V8 juice, Difco PDA 10 g and dH2O to 1 L) (Carlsen et al. 2017). Afterward, the culture was then incubated for 5 days in the dark at 25 °C. This was followed by an additional incubation period with a 12-h light/dark photoperiod for a total incubation period of 6 days.
The Z. tritici strain ST38, characterized by its law resistance to triazole fungicides (TriR3), was kindly provided by Dr. Sameh SELIM (UniLaSalle, Beauvais, France). This strain was isolated in 2005 in France from the Orvantis wheat variety from a sporiferous cirrhe (Samain et al. 2022). The fungus was cultivated on PDA medium and incubated at 20 °C with a 12 h/12 h photoperiod for 5 days to induce spore production.
Wheat assay was performed using the cultivar Lennox (2012) from Saaten Union (Estrées-Saint-Denis, France), provided by le Groupe Soufflet (Nogent-sur-Seine, France). This spring variety exhibits a Z. tritici resistance level of 4.5 according to Arvalis’ scale (1: high susceptible, 9: high resistant). Regarding the P. teres MUCL 28818 experimentation, the barley cultivar KWS Fantex (Einbeck, Germany), provided by le Groupe Soufflet, was employed. This cultivar is a 2-row variety with a sensibility to P. teres of 5. Seeds were sown in 9 × 9-cm pots containing GramoFlor soil (F05) (Vechta, Germany), and incubated in a growth chamber at 20 °C (+ / − 2 °C) with 80% humidity for all assays. The primers used in this study are listed in Supplemental Table S1.
Direct effect on the pathogen development
To assess the direct impact on pathogen development, dual culture assays were used to evaluate the effect of strain BE2 (DSM 115797) against both Z. tritici ST38 and P. teres MUCL 28818. After 5 days of incubation, spores from Z. tritici ST38 were harvested by gently scraping the surface of the fungal culture using a sterile 10 mM of MgSO4 buffer and subsequently adjusted to a concentration of 106 spores/mL using a Malassez counting chamber. Next, 500 µL of this spore suspension were evenly spread onto the surface of a PDA plate. The plate was subsequently incubated at 25 °C in the dark. After 24 h of incubation, 5 µL of the strain BE2 suspension (adjusted to a concentration of 108 colony-forming unit per millilitres (CFU/mL)) was deposited in the center of the plate, followed by re-incubated at 25 °C for 7 days.
For P. teres MUCL 28818, a disk containing P. teres mycelium was placed at the center of a PDA plate. The plate was incubated for 2 days at 25 °C in the dark to allow the development of the fungus. Then, 5 µL of the BE2 suspension (108 CFU/mL) were spotted at three equidistant places on the plate borders. These plates were re-incubated for a period of 3 to 7 days under the same conditions as mentioned above. For both pathogens, plates inoculated with the fungus alone were also prepared as positive control. The antifungal effect was evaluated by measuring the diameter of mycelium growth on plates containing BE2 in comparison to the control plates after 7 days of confrontation. Each experiment was independently repeated three times in triplicate. Furthermore, to gain a detailed understanding of the inhibitory effect of strain BE2 on hyphal growth and morphology in both pathogen, mycelial samples from both pathogens were observed under a 3D microscope (Keyence, France) after one week of confrontation.
Pyrenophora teres bioassay on detached leaves
Detached leaf assay was carried out to evaluate the biocontrol activity of strain BE2 against P. teres infection, following the protocol previously described by Decouard et al. (2022). Briefly, barley plants were placed in a growth chamber equipped with an ultrasonic humidifier labelled as “2017 generation” (ARALAB, Rio de Mouro, Portugal). The growth chamber was set at a temperature of 20 °C, with a 12-h photoperiod and 80% relative humidity (RH). When the barley plant reached the 3-leaf stage (3-L stage), which occurred approximately 14 days after planting, the second leaf was carefully removed. A 7-cm central segment of the detached leaf was disinfected by immersing it in a 70° ethanol bath for 30 s, followed rinsing in three consecutive sterile water baths. Subsequently, these leaves were then placed on agar supplemented with kinetin medium (per liter: 10 g agar + 10 mL kinetin at 1 g/L). To facilitate inoculation and allow the development of the fungus and the appearance of symptoms, the leaves were superficially injured at the point of inoculation using a sterile toothpick. Each leaf was then inoculated in the center of the upper surface with 5 µL of the spore suspension containing 5000 spores/mL of P. teres. After the first drop containing the pathogen had dried, 5 µL of the bacterial suspension (108 CFU/mL) were added at the same point of inoculation. For the negative control, 5 µL of sterile H2O was applied at the leaf wound area while for the positive control, 5 µL of P. teres spores’ suspension was inoculated at the leaf wound site. Additionally, the product Vacciplant®, known for its strong activity against P. teres infection, was used as a control reference. Petri plates containing the treated leaves were then incubated under the same conditions as previously described for a period of two weeks. After this incubation period, the size of necrosis was measured at 10 days post-infection (dpi). Each experiment was replicated three times, and all experiments were independently repeated three times for robustness and reliability.
Whole-genome sequencing and in silico analysis
To comprehensively explore all potential gene clusters linked to the biocontrol activity, the whole genome of strain BE2 was sequenced at Microbes NG (Birmingham, UK), accessible at http://www.microbesng.uk. This sequencing process utilized Illumina MiSeq and HiSeq 2500 technology platforms, employing a library with 2- 250-bp reads. De novo assembly was performed using the SPAdes assembler. Gene annotation and open reading frames (ORFs) prediction were carried out by submitting the whole genome draft sequence of BE2 to the Bacterial and Viral Bioinformatics Resource Center (BV-BRC) (https://www.bv-brc.org/) (Olson et al. 2023). The construction of the phylogenetic tree was based on the whole genome sequence using the codonTree method within BV-BRC. To investigate the presence of potential genes associated with secondary metabolites synthesis, especially those involved in non-ribosomal peptides and polyketides synthesis, the draft genome sequence of strain BE2 was subjected to analysis using the online antiSMASH software, accessible at https://antismash.secondarymetabolites.org/#!/start) (Blin et al. 2021). The draft genome sequence of strain BE2 has been deposited in GenBank under the accession number RRZG00000000. The version described in this paper is version RRZG01000000.
Antifungal activity of BE2 culture supernatant against P. teres and Z. tritici
The BE2 strain was grown in Landy medium and incubated at 30 °C with a constant shaking (180 rpm) for 48 h. Subsequently, the bacterial culture was centrifugated twice at 10,000 g for 10 min at 10 °C. The resulting supernatant was then filtered through a 0.2-μm filter. This supernatant was evaluated for its ability to inhibit fungal development and spore germination through both liquid and solid assays.
For the solid assay, twenty-four well-plates were filled with 1.5 mL of PDA previously mixed with varying proportions of the supernatant to achieve final percentages of 0, 0.5, 1, 1.5, 2, and 3%. Subsequently, plates were inoculated with 5 µL of spores at a concentration of 106 spores/mL and 5.103 spores/mL for Z. tritici ST38 and P. teres MUCL 28818, respectively. The plates were then incubated in the dark at 25 °C for 7 days.
In the liquid assay, sterile 96 well-plates containing 200 µL of PDB with the same concentrations of supernatant and spores’ pathogens as previously described, were incubated in a TECAN® Microplate reader (Infinite F200 PRO, Tecan, Männedorf, Switzerland) at 25 °C with continuous shaking (132 rpm). The antagonistic activity of the supernatant was assessed by measuring the absorbance at 620 nm every 6 h for 3 days. For the control, cycloheximide at a final concentration of 100 µg/mL was used in both assays. Three replicates (wells) were assessed for each condition and the experiment was carried out in triplicate. Microscopic observation of Z. tritici ST38 and P. teres MUCL 28818 spores was visualized at 24 h, 48 h, and 72 h post-inoculation (hpi) with an inverted microscope EVOS FL Auto Imaging System (Thermo Fisher Scientific, Waltham, MA, USA).
A detached leaf assay with the supernatant was performed using the same method as previously described (Decouard et al. 2022). In this assay, leaves were superficially injured on the upper side, and 5 µL of P. teres MUCL 28818 at a concentration of 5000 spores/mL was applied. Once the drop dried, 5 µL of the supernatant solution was added. Leaves inoculated with only Landy medium or spores’ suspension served as negative and positive control, respectively. The plates were then incubated in a culture chamber at 20 °C with 80% RH. After 7 days, the size of necrosis was measured. This experimentation was repeated three times in three independent replicates.
UPLC-MS analysis of strain BE2 filtrate
The BE2 strains was cultured in LB at 30 °C with constant shaking at180 rpm for 48 h. Following this, the supernatant was collected and filtrated using a hydrophilic syringe filter (0.20 µm). Subsequently, the cell-free supernatants from strain BE2 were analysed by UPLC-qTOF MS. Strain BE2 metabolites were identified using an Agilent 1290 Infinity II coupled with mass detector (Jet Stream ESI-Q-TOF 6530) in positive mode with the parameter set up as follows: capillary voltage of 3.5 kV, nebulizer pressure of 35 psi, drying gas of 8 L min−1, gas temperature of 300 °C, flow rate of sheath gas of 11 L/min, sheath gas temperature 350 °C, fragmentor voltage of 175 V, skimmer voltage of 65 V, and octopole radio frequency of 750 V. Accurate mass spectra were recorded in the m/z range of 100 to 1700 (acquisition rate 2 spectra/s). A C18 Acquity UPLC BEH column (2.1 mm; 50 mm; 1.7 µm; Waters, Milford, MA, USA) was used at a flow rate of 0.6 mL/min and a temperature of 40 °C (injection volume: 5 µL). A gradient of acidified water (0.1% formic acid) (solvent A) and acidified acetonitrile (0.1% formic acid) (solvent B) was used as mobile phase with a constant flow rate at 0.6 mL/min starting at 10% B and rising to 100% B in 20 min. Solvent B was kept at 100% for 4 min before going back to the initial ratio. The different metabolites were identified based on retention time, by comparison with in-house database constructed with mutants impaired in the production of the different metabolites (Andrić et al. 2023), and accurate mass. The iturin structure was confirmed by MS/MS fragmentation of two precursor ions at m/z = 1043.55 and 1057.57 using 40 V as collision energy.
Siderophore activity
In order to evaluate the ability of strain BE2 to produce siderophore, the strain was cultivated in an iron-deficient minimum medium (MM9). To assess siderophore production, a CAS (chrome azurol S) agar assay was performed following the methodology described by Esmaeel et al. (2016). Positive results were indicated by the formation of a distinct, clear orange zone around the bacterial colonies, showing a visible change from the original blue to either yellow or orange.
Biofilm formation
The biofilm production of strain BE2 was evaluated following a modified version of the method described by Rondeau et al. (2019). Initially, a pre-culture of the bacterium in LB medium was diluted to an OD600 of 0.2. Then, 500 µL of the diluted culture were placed in polystyrene tubes and incubated at 28 °C under static conditions of 24 and 48 h. Following incubation, the excess medium was removed, and the tubes were carefully rinsed with H2O. Adherent bacteria were then stained with a 1% crystal violet solution, and the tubes were left at room temperature for 10 min. After that, tubes were rinsed five times with H2O and were allowed to air-dry at room temperature. Subsequently, the crystal violet bound to the biofilm was solubilized in 1 mL of 30% acetic acid for 10 min. The absorbance of the resulting solution was measured at 570 nm using spectrophotometer. Each assay was performed independently three times in triplicate.
Protection of wheat and barley plants
To evaluate the efficacy of strain BE2 against Z. tritici ST38 and P. teres MUCL 28818 in planta, two different modes of application were employed, including foliar spray and root drenching.
For foliar application, at the 3-L stage, plants were sprayed with a 3 mL of BE2 suspension at a concentration of 108 CFU/mL. Three days after the bacterial inoculation, plants were infected by spraying with 3 mL of fungal strain Z. tritici ST38 suspension (106 spores/mL) or P. teres MUCL 28818 (5000 spores/mL) suspended in 10 mM of MgSO4 containing 0.05% of Tween 20.
For root application, seeds were sterilized and pre-germinated as described by Selim et al. (2014). Briefly, grains were disinfected by incubation in a 1% sodium hypochlorite for 5 min with agitation and then washed three times with sterile distilled water. Subsequently, grains were transferred and incubated in 0.5% water-agar medium for 24 h at 20 °C, then at 4 °C for 48 h, and finally at 20 °C for 24 h. The wheat grains were then immersed in a suspension of strain BE2 adjusted to 108 CFU/mL in 10 mM MgSO4 at a ratio of 1 mL per grain. They were left for 1 h with gentle shaking. Finally, grains were sown under the same conditions as described for foliar application. One week before pathogen infection, plants were inoculated for a second time with 1 mL of BE2 suspension per plant at the root level.
As controls, plants were watered with 10 mM of MgSO4 (negative control), while others were infected with Z. tritici ST38 or P. teres MUCL 28818 alone (positive control). Reference products, HELIOTERPEN® SOUFRE (Helioterpen, Castets, France) and VACCIPLANT® (GoËmar, Saint-Jouan-des-Guérets, France) were also applied for Z. tritici ST38 and P. teres MUCL 28818, respectively.
Following infection, plants were covered with transparent plastic bags to maintain 100% humidity for 3 days. The percentage of necrosis was determined at 7, 17, 21, and 25 days post-infection (dpi) for Z. tritici ST38 and at 3, 5, 7, and 10 dpi for P. teres MUCL 28818. The experiment was replicated three times in three independent repetitions.
Pathogen detection in planta
To assess the presence and progression of pathogens in planta, various analyses were conducted, including visual symptom notation and fungal DNA quantification by qPCR.
For visual notation, symptoms were visually assessed by calculating the percentage of chlorosis on the third leaf at specific time points, specifically 7, 17, 21, and 25 dpi for Z. tritici ST38 and 3, 5, 7, and 10 dpi for P. teres MUCL 28818.
For the quantification of fungal DNA, the third leaf was collected, freeze-dried in liquid-nitrogen, and stored at − 80 °C before fungal DNA extraction. For the extraction of fungal DNA of Z. tritici ST38, two leaves were grounded for each modality at each sampling time points. Total DNA was extracted from 50 mg of leaf powder extracted using the purification DNA Kit (Promega Corp., Madison, WI, USA) following the manufacturer’s instructions. DNA quality was checked by agarose gel electrophoresis, and the DNA concentration was measured at 260 nm using a NanoDrop (Thermo fisher scientific, Illkirch-Graffenstaden, France). The DNA concentration was adjusted to 100 ng/µL. The quantification of the fungal DNA was performed using qPCR following the protocol described by Bearchell et al. (2005). The real-time quantitative PCR targeted the Mycosphaerella graminicola β-tubulin (GenBank accession no. AY547264). This gene exhibits variability among species, providing a high level of specificity, and is not repeated in cells, making the number of β-tubulin gene copies equivalent to the number of fungal cells (Fraaije et al. 1999).
Real-time qPCR reactions were carried out in duplicate in 96-well plates, each containing a final volume of 25 μL. The reaction mix consisted of 12.5 µL of Universal Taqman PCR Master Mix (Life Technologies SAS, Villebon, France), 0.2 µM of the probe (FAM-TTACGCCAAGACATTC-MGB), 0.3 µM of each forward (5′-GCCTTCCCCCACCAT-3′) and reverse (5′-CCTGAATCGCATCGTTA-3′) primers, 200 ng of DNA, and sterile nuclease free H2O to bring the total volume of 25 µL, followed the protocol outlined by Samain et al. (2019).
For the quantification of P. teres MUCL 28818, total DNA was extracted from 100 mg of leaf powder with the cetyltrimethylammonium bromide (CTAB) buffer protocol following the protocol outlined by Ghaffariyan et al. (2012). DNA quality and quantity were checked as previously described. The quantification of the fungal DNA was conducted via qPCR, following the protocol described by Leisova et al. (2006). TaqMan probes and primers were designed based on DNA sequence differences between P. teres f. teres and P. teres f. maculate, with the probes specific to P. teres were labelled with FAM fluorescent dye. Real-time PCR reactions were carried out in duplicate within 96-well plates, each containing a final volume of 25 μL. This volume included 12.5 µL of Universal TaqMan PCR Master Mix (Life Technologies SAS, Villebon, France), 0.2 µM of the FAM probe (FAM-AGTTGTTTGTGGTTTTC-MGB), 0.3 µM of each Forward (5′-TACACCTACGCTTGACGCATG-3′) and Reverse (5′-GATATCTTCATCTGCGGACCG-3′) primers, 250 ng of DNA and water to reach a volume of 25 µL.
Both quantification assays employed the same qPCR cycles conditions: an initial denaturing step at 95 °C for 10 min, followed by 40 two-step cycles of 15 s at 95 °C and 1 min at 60 °C, using the CFX-Touch BioRad96 (Hercules, CA, USA). The amount of DNA was calculated using a standard range spanning from 102 to 107 copies of the gene, created through serial dilution of the appropriate cloned target sequence. Prior to analysis, the primer specificity was verified through PCR and qPCR with strain BE2 and plant leaves without pathogen. The final DNA concentration was expressed per 100 ng of total DNA in each sample and data analysis was performed using CFX-Maestro™ Software v2.2 (BioRad, Hercules, CA, USA).
Potential of strain BE2 to modulate the expression of plant defense gene markers against Z. tritici and P. teres
Grain sterilization, pre-germination, inoculation with strain BE2, and the respective pathogen, as well as plant growth conditions were carried out as mentioned above. At the fifth leaf stage, the third leaf was collected at the infection time (T0). Then, for Z. tritici, the expression of defense genes was evaluated at 6, 12, and 24 hpi, as well as at 3 and 5 dpi. In the case of P. teres, the third leaf was collected at 6, 12, nd 48 hpi, along with 3 dpi. Each sample represents two leaves of two distinct plants and was promptly placed in liquid nitrogen before being stored at − 80 °C. RNA extraction was carried out using 100 mg of leaf powder, and the subsequent cDNA synthesis utilized EXTRACT-ALL (Eurobio, Les Ulis, France) and Verso cDNA (Thermo fisher scientific, Illkirch-Graffenstaden, France), following the manufacturer’s guidelines. RNA quality and quantity were checked by agarose gel electrophoresis and the NanoDrop (Thermo fisher scientific, Illkirch-Graffenstaden, France) respectively. SYBR Green qPCR assays were carried out in 15 μL reaction mixture, which included 7.5 μL of 2 × qPCRBIO SyGreen Blue Mix (PCR Biosystems Ltd, London, UK), 1.4 μM of primer mix, 1.1 µL of nuclease-free water, and 5 µL of cDNA. The qPCR conditions were as follows: 3 min at 95 °C, followed by 40 cycles of 5 s at 95 °C and 20 s at 60 °C with a final step from 60 to 95 °C to confirm primer specificity. Each modality represents 6 plants, and three independent replicates were performed.
The target genes are presented in Supplemental Table S1. The expression levels were normalized using two housekeeping genes for each plant condition. For the wheat assay, the two genes used were Act for actin (AB181991) and AP-5 for complex subunit mu-like CJ705892 (XM_044539803.1). In the barley experimentation, Act (AY145451) and ribosomal protein S4 RP-S4 (NC_042692.1) were selected as the housekeeping genes.
Root colonization assay
The root colonization assay aimed to assess the colonization of wheat and barley roots by strain BE2 using a modified protocol based on the method described by Issa et al. (2018). Initially, strain BE2 was rendered resistant to 25 µg/mL of chloramphenicol through successive transplants on LB medium with varying antibiotic concentrations. Subsequently, wheat and barley grains were subjected to sterilization and inoculation procedures as previously mentioned in “Pathogendetection in planta.” Additionally, GramoFlor soil (F05) underwent double sterilization to limit the potential influence of microorganism interactions with strain BE2. For epiphyte colonization, root samples were retrieved from the soil and placed in sterile tubes containing 3 mL of sterile PBS, followed by vortexing for 3 min at 240 rpm. The resulting mixture was serially diluted with sterile H2O in tenfold dilutions, and then, 100 µL from the appropriated dilution was cultured in triplicates on LB medium supplemented with chloramphenicol (25 µg/mL). To determine the endophytic colonization, roots and shoot (stem and leaves) were surface disinfected. This entailed a 3-min immersion in 70% ethanol (1 min for leaves), followed by a 1% commercial bleach + 0.01% Tween 20 solution for 1 min, and subsequently, three rinses with sterile distilled water. The samples were then grounded and homogenized with 1 mL of sterile water. The resulting homogenate was serially diluted and spread as described earlier. To validate the efficacy of the sterilization process, 100 µL of the final water rinse were placed on three LB plates containing chloramphenicol (25 µg/mL). These plates were then incubated at 28 °C for 48 h, and the colony count was expressed as the mean of colony-forming units (CFU) per gram of fresh root or shoot weight (stem and leaves). Both external and internal colonization dynamics were investigated at various time points post-bacterization (0, 1, 7, 14, and 21 dpi). Each treatment, including non-inoculated plants and BE2-treated plants, was conducted in triplicate for both technical and biological replicates.
Statistical analysis
Statistical analyses were carried out using Rstudio software version 4.1.1 (https://cran.r-project.org/bin/windows/base/old/4.1.1/). The normality of the data was tested via Shapiro test and Kruskal test used to study the correlation of beneficial bacterium with P. teres MUCL 28818 and Z. tritici ST38. The graphics were realized using the software GraphPad Prism version 9.0.0 (Boston, MA 02110, USA) (https://www.graphpad.com/features).
Results
Identification, genomic insights, and secondary metabolic profiling of strain BE2
Strain BE2 was identified as B. velezensis BE2 based on its 16S rRNA gene sequence and morphological traits. Additionally, phylogenetic analysis using the whole genome sequence confirmed its placement within the Bacillus genus, showing a close relationship to the B. velezensis clade with a robust bootstrap score of 100% (Fig. 1). The genome of strain BE2, assembled into 35 contigs with an N50 value of 622,732 bp, and L50 value of 2, has a total size of 4,210,155 bp and maintains an average G + C content of 45.85% (Fig. 2A). Within this genome, a total of 4254 protein coding genes were identified. Among these, 3420 genes were functionally annotated, while 966 were assigned as hypothetical proteins. The predicted gene products exhibited an average length of 300.6924 amino acids and a coding density of 89.1118%. These coding genes were distributed across 469 subsystems, with a notable presence in functions related to carbohydrate transport and metabolism (5.35%), amino acids and derivatives synthesis (6.40%), defense mechanisms (1.52%), coenzyme transport and metabolism (2.71%), and the biosynthesis and transport of secondary metabolites (2.25%). The distribution and counts of genes in clusters of orthologous groups (COGs) categories for the genome of strain BE2 are presented in Fig. 2B.
Phylogenetic tree analysis based on the whole-genome sequence, illustrating the taxonomic position of strain BE2 within the Bacillus genus. Bootstrap values, indicative of the confidence in the branching points, are displayed on the tree. The tree was constructed with The Bacterial and Viral Bioinformatics Resource Center (BV-BRC) (https://www.bv-brc.org/) and visualized using ITOL (https://itol.embl.de/)
A Circular genome viewer of strain BE2 genome. Starting from the outmost circle and moving inwards, each ring represents different genome features, including contigs, forward CDSs, reverse CDSs, RNA genes, transporters, GC content, and GC skew. The circular genome visualization was generated using the circular viewer of the BV-BRC ((https://www.bv-brc.org/). B Clusters of orthologous groups showing the functional classification of genomic protein of the BE2 genome. Clusters were predicted using the Eggnog database via MicroScope platform (https://mage.genoscope.cns.fr/microscope/home/index.php). Letters represent function classes, which are presented on the right side of the graph. The data on the vertical axis indicates the number of CDS associated with each functional class. C The Venn diagram represents the core-genome, variable-genomes, and strain specific sizes. The values displayed on the figure represent the number of gene families for each organism intersections
For further insights, a comparative analysis involving strain BE2 and the three reference Bacillus strains, including Bacillus amyloliquefaciens subsp. plantarum AS43.3, B. velezensis YAU B9601-Y2, and B. velezensis FZB42 was conducted (Fig. 2C). Analysis of the CDSs (coding sequences) of the four strains revealed a total of 3293 genes that were common to all of them. Additionally, strain BE2 shared 3509 and 3614 genes with FZB42 and YAU B9601-Y2, respectively (Fig. 2C).
To gain a deeper understanding of strain BE2’s potential biocontrol activity, an extensive analysis of its genome was undertaken to identify the presence of secondary metabolite gene clusters. The analysis pointed out the presence of 15 different secondary metabolite gene clusters with diverse potential activities. Figure 3 highlighted the most similar clusters identified using antiSMASH, while a comprehensive list of predicted metabolites is available in Supplemental Table S2. The in silico analysis predicted the presence of many non-ribosomal peptides (NRPs) and polyketides (PKs) gene clusters that are likely involved in the synthesis of various products, including surfactin, fengycin, iturin, difficidin, rhizocticin, butirosin, macrolactin, bacillaene, bacillibactin, and bacilysin.
Biosynthesis gene clusters of different NRPS–PKS secondary metabolites predicted in the genome of strain BE2. A: adenylation domain; C: condensation domain; CP: carrier protein domain; E: epimerization domain; TE: thioesterase domain; KS: ketosynthetase; AT: acetytransferase; KR: ketoreductase; DH: dehydratase; M: module. The percentage of identity of each of these metabolites was compared to those produced by other strains, including B. velezensis YAU B9601-Y2; B. amyloliquefaciens DSM 7; B. amyloliquefaciens subsp. plantarum AS43.3 and B. velezensis FZB42
Antifungal activity against Z. tritici and P. teres
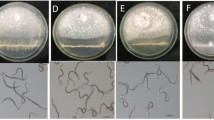
The antagonism potential of strain BE2 against Z. tritici and P. teres was evaluated through direct confrontation tests on PDA medium. Remarkably, strain BE2 significantly reduced the growth and development of the pathogen P. teres MUCL 28818 by up to 75%. Indeed, within just 4 days, the bacterium completely halted the growth of P. teres. This is evident when comparing the color of the fungus in Fig. 4A to that on the control plate. Initially, the fungus appeared pale, transitioning to an orange along the areas where it confronted the bacterium, and eventually turning a dark orange shade. To comprehensively investigate the inhibitory effect of strain BE2, we compared the hyphal growth and morphology of the pathogen in confrontation with the bacterium to that in the control. Microscopic observation showed that the hyphae in the control developed across the entire medium without inducing many ramifications and maintained a relatively linear structure. Conversely, when the fungus encountered the bacterium, the hyphae formed significantly more branching, and a beginning of sporulation were observable at 3 dpi (Fig. 4B).
Antagonism assay of the beneficial strain BE2 DSM 115797 against Z. tritici and P. teres. A Visual observations of the effect of strain BE2 on P. teres MUCL 28818 after one week (1. pathogen alone and 2. pathogen against strain BE2). B Microscopic observations (× 100) of the interface between P. teres MUCL 28818 and strain BE2 after one week (1 and 2: P. teres without and with interactions against strain BE2, respectively). C Visual observations of strain BE2 effect against Z. tritici ST38 after 5 days (1. pathogen alone and 2. pathogen against strain BE2). D Microscopic observations (× 60) of the interface between Z. tritici ST38 and strain BE2 after one week (1. pathogen alone and 2. pathogen against strain BE2). E Visual observations of detached leaves assay inoculated with strain BE2 one-week post infection with P. teres. Leaves were disinfected and injured, and then, 5 µL of a solution containing the beneficial bacterium and 5 µL of P. teres MUCL 28818 were applied to the injured area. For controls, a reference product named Vacciplant® was used. A positive control and a negative control were realized with inoculation of the pathogen alone or with H2O respectively. Plates were then incubated at 20 °C with a photoperiod of 12 h/12 h and the length of the necrosis was measured one-week post infection
The beneficial bacterium strain BE2 also had a direct impact on the development of the fungus Z. tritici ST38 (Fig. 4C) and may imply different mechanisms to compete with this pathogen. When confronted with strain BE2, the fungus exhibited a visible halo, indicative of fungus lysis. Similarly, to its interaction with P. teres, strain BE2 triggered the formation of Z. tritici spores (depicted as black spots) around the inhibition zone, attributed to a stress reaction from the pathogen occurring after 4 dpi, a phenomenon further confirmed through microscopic observations (Fig. 4D). Consequently, with both pathogens, the bacterium showed a good capacity to inhibit the growth of both fungi in vitro.
The ability of strain BE2 to control net blotch disease in barley was also evaluated on detached leaves. As show in Fig. 4E, the BE2 suspension exhibited a good efficacy against P. teres. Two days post infection, P. teres-related necrosis started to be visible in leaves of the positive control, and one week later, the fungus had spread several centimeters beyond the leaf wound. Conversely, no symptoms were observed in leaves treated with the control product Vacciplant®. Leaves treated with strain BE2 and subsequently infected with P. teres displayed smaller lesion or, in some cases, no symptoms at all when compared to the product reference control.
Direct antifungal activity of BE2-free supernatant
The potential of strain BE2 to produce secondary metabolites with antagonistic activities on different pathogens was evaluated using the BE2-free supernatant against Z. tritici and P. teres. The supernatant exhibited strong antifungal activity against both pathogens, displaying a dose-dependent response across all tested percentages (Supplemental Fig. S1).
Spore germination inhibition was evident in the case of Z. tritici ST38. While a gradient of inhibition was observed in relation to the percentage of the supernatant, the bacterial supernatant consistently exerted significant interference with spore germination. Remarkably, Z. tritici was almost impaired by the culture supernatant, with germination reduced by half when the liquid assay medium contained just 1% of the supernatant (Fig. 5A). Furthermore, the culture filtrates completely inhibited spore germination in P. teres across all tested concentrations (Fig. 5B). Microscopic observation provided additional confirmation of the anti-germinative effect of the BE2-free supernatant (Supplemental Fig. S2).
A Z. tritici and B P. teres growth in the presence of different percentages of cell-free BE2 supernatants. The growth was assessed by measuring the absorbance at 620 nm at every 6 h during incubation using TECAN® Microplate reader (Infinite F200 PRO, Tecan, Männedorf, Switzerland). C Visual observations of detached leaves assay inoculated with strain BE2-free supernatant one-week post infection with P. teres. Leaves were disinfected and injured, and then, 5 µL of a solution containing the supernatant and 5µL of P. teres MUCL 28818 were applied to the injured area. Plates were then incubated at 20 °C with a photoperiod of 12 h/12 h and the length of the necrosis was measured one-week post infection
In detached leaf assays, the supernatant from the bacterium was tested against P. teres in detached leaves assays. The efficacy was comparable to that observed in previous experiments realized with the culture of strain BE2. Notably, there was a significant reduction in the size of necrosis arears when leaves were inoculated with the supernatant (Fig. 5C).
Strain BE2 produces different families of cyclic lipopeptides potentially involved in biocontrol
Based on molecular ion masses, seven non-ribosomally produced families of metabolites were identified (Fig. 6A). This analysis highlighted the significant production of three very well-characterized cyclic lipopeptides families commonly produced by several Bacillus species (Fig. 6B). Specifically, strain BE2 produced molecules with m/z values corresponding to analogues of iturin, fengycin, and surfactin family with various fatty acid chains. As iturin shares a common mass with other family members, such as mycosubtilin, structural confirmation was performed by targeted MS/MS analyses. Additionally, strain BE2 was found to produced different types of polyketides, including macrolactins (A/D/malonyl/succinyl), bacillaenes (bacillaene and dihydro-bacillaene), difficidins (difficidin and oxy-difficidin), and the siderophore bacillibactin (Supplemental Table S3). Furthermore, the strain’s capability to produce the iron-chelator bacillibactin was confirmed with optimal environment conditions using CAS medium (Supplemental Fig. S3).
A LC–MS analysis spectra of the free culture supernatant of strain BE2. The intensity (y-axis) was as a function of the retention time (x-axis). Secondary metabolites produced by the strain were identified as follows: surfactins, fengycins, iturins, bacillaenes, difficidins, macrolactins and bacillibactin. The grey zone represents to peak area of selected peaks (with the value above the peak). B Chromatogram by UPLC-MS of the three main cyclic-lipopeptides. The signal intensity TIC (y-axis) is plotted as a function of the retention time (min)
Rhizosphere colonization by BE2
In order to gain a deeper understanding of how the beneficial bacterium BE2 interacts with plants, epiphytic and endophytic colonization of wheat and barley was carried out at various intervals following bacterization. The objective of the trial was to ascertain if the internal tissues of the plant could be penetrated and colonized by the beneficial bacterium. When the various parts of the disinfected plant were crushed, it was observed that no colonies were growing (data not shown), indicating that the tissues had not been penetrated by the bacterium and that the bacterium was therefore not endophytic.
Furthermore, following the characterization of the bacterium’s endophytic or epiphytic nature, the investigation of whether the bacterium remained in contact with the plant during its growth up to the stage of pathogen inoculation was deemed important. The results showed that the rhizosphere space of wheat and barley was colonized by the strain, even if it was not present inside the plant tissues. Additionally, the bacterium was found to be capable of surviving in the soil and at the root interface through the period of plant infection, spanning the development and transition of the pathogen to the necrotrophic phase (Supplemental Fig. S4). Although concentrations gradually decreased during the trial, declining from 108 CFU/g to 105 CFU/g of root, the beneficial bacterium was still detected after 21 dpi, corresponding to the onset and development of Z tritici symptoms. In parallel, it was demonstrated through crystal violet assays that the strain has a good capacity to form a biofilm, thereby enabling colonization of wheat and barley roots (Supplemental Fig. S4).
BE2 protects wheat and barley against their pathogens
To further explore the biocontrol properties and the ability of strain BE2 to trigger ISR for defending wheat against Septoria tritici blotch, two different application methods (foliar and drenching) were employed. This allowed for the optimization of strain BE2’s efficacy and a deeper understanding of the protection mechanism in the tripartite interaction between the plant, the bacterium, and the fungus. Disease progression was assessed at 25 dpi. Results showed that strain BE2 significantly protected wheat plants, resulting in an average 52% reduction in disease symptoms compared to the disease control (Fig. 7A1).
Visual observations of wheat leaves at 21 dpi (A.1). The negative control (NC): plants inoculated with MgSO4 10 mM; the positive control (PC): plants sprayed with a solution of the pathogen Z. tritici ST38 (concentration 106 spores/mL) at the three leaf growth stage, the modalities with BE2 root (BE2-R) and foliar (BE2-F) were plants infected by the pathogen but also inoculated with a solution of beneficial bacterium (concentration 108 CFU/mL) at the root and foliar levels, respectively. Plants were incubated in a grown chamber at 20 °C (+ / − 2 °C) with 80% humidity. A.2 Detection of Z. tritici ST38 DNA in planta by qPCR. Statistical analyses were realized at 25 dpi using Tukey test (p ≤ 0.05). B.1: Visual observations of barley leaves at 10 dpi. The negative control (NC), inoculated with a solution of MgSO4 10 mM; the positive control (PC), plants were sprayed with a solution of the pathogen P. teres MUCL 28818 (concentration 5000 spores/mL) at the three leaf growth stage, the modalities with strain BE2 root (BE2-R) and foliar (BE2-F) are plants infected by the pathogen and inoculated with a solution of beneficial bacterium (concentration 108 CFU/mL) at the root and foliar levels, respectively. Plants were incubated under the same conditions as the wheat plants. B.2 Detection in planta of the P. teres MUCL 28818 DNA by qPCR. Statistical analyses were realized at 10 dpi using Tukey test (p ≤ 0.05). In both qPCR assays, the final concentration was normalized per 100 ng of total DNA in each sample. The values presented are the means of three biological replicates
However, this analysis method was based on visual annotation and did not account for the possibility of the fungus being present in the leaves without inducing visible symptoms. To mitigate this visual annotation bias, fungal growth was monitored in planta at 3, 7, 17, 21 and 25 dpi through qPCR for fungal DNA quantification. Findings indicated that strain BE2 hindered pathogen development, but the effect depended on the mode of application (Fig. 7A2). After 25 days, the fungus had developed in all inoculated modalities with Z. tritici ST38, but an influence of strain BE2 was still evident. In the initial days, foliar application of the bacterium was the most effective in curtailing pathogen proliferation. However, after 14 dpi, the pathogen concentration increased exponentially, suggesting that the bacterium’s effectiveness waned. On the contrary, with root application, the initial pathogen concentration was higher during the early days, but the increase was more progressive. Finally, at 21 dpi, coinciding with the appearance of symptoms, the fungal DNA concentration was significantly lower than in the control. Thus, strain BE2 manged to reduce the pathogen load by 40,000 copies of fungal DNA per 100 ng/µL of total DNA compared to the control.
Regarding the reduction of net blotch symptoms, a similar in planta assay was conducted, whether by foliar application or drenching. Visual observations at 10 days after infection showed that strain BE2 significantly reduced disease symptoms on barley leaves (Fig. 7B1). Additionally, the most effective application was at the root level, resulting in up to 25% reduction in leaf symptoms. Similar to the Z. tritici ST38 assay, DNA quantification of the P. teres MUCL 28818 in planta at 3, 5, 7, and 10 dpi illustrated the impact of strain BE2 (Fig. 7B2). The development of the P. teres pathogen was notably reduced when plants were inoculated with strain BE2 at the root level after 10 dpi. Within three days, the pathogen concentration was significantly lower compared to other modalities. Between the fifth and the seventh day after infection, there was a slight increase in concentration, but a robust inhibition was observed starting from the first week. Consequently, strain BE2 exhibited an effect as significant as the reference product but, unlike the registered product, its effect was stronger after 10 days.
BE2 modulates different defense markers against Z. tritici and P. teres in wheat and barley
Prior experiments established that strain BE2 exhibited a strong capability to reduce disease symptoms and the development of the Z. tritici and P. teres pathogens in wheat and barley, particularly when applied at the root level. To delve into strain BE2’s potential to induce defense mechanisms in plants, the expression of defense-related genes (CHIT, POX, PR1, GLU, and FLAV for wheat and LOX2, PAL2, AOC, GSL3, GST, PR1, and PR5 for barley) was investigated (Supplemental Table S1). These genes are known to respond to different signaling pathways. The investigation was conducted at 6,12, and 24 hpi and 3, 5 dpi for Z. tritici and 6, 12, 48, and 72 hpi for P. teres, respectively. As depicted in Figs. 8 and 9, the expression of these genes displayed significant variation in the period following pathogens infection. When plants were infected with the Z. tritici ST38 fungus, genes were significantly over-expressed compared to non-infected controls (Fig. 8). However, in plants that were inoculated with strain BE2 and then infected with the pathogen, gene expression was significantly higher than in plants infected with the pathogen alone. The genes PR2 and PR3, encoding the hydrolytic enzymes of β-1,3-glucanase (GLU) and chitinase (CHIT), were both over-expressed during the first hours following infection. However, their expressions did not significantly differ from the positive control at other time points. Similarly, the POX gene was also over-expressed at 12 and 24 hpi. The FLAV gene, responsible for the production of antifungal compounds, showed overexpression at 6, 12, and 24 hpi. Additionally, at 6, 12, and 24 hpi, the PR1 gene, known to be a marker of SAR and for its antimicrobial activity (Ali et al. 2018), also displayed overexpression. Thus, it was evident that the selected five genes, serving as markers for inducing defense responses in the plant, were significantly more expressed during the first hours after pathogen infection compared to other modalities. Furthermore, controls inoculated with the bacterium alone did not modulate the gene expression except during the very early stage (6 hpi).
Time course of the relative expression of wheat defense genes in the cultivar Lennox after at 6, 12, 24 hpi, as well as 3 and 5 dpi with Z. tritici infection. Plants were inoculated with strain BE2, by immersing the pre-germinated grains in a suspension of 10.8 CFU/mL. Gene expression was assessed in various experimental conditions, as represented by different bar colors in the chart: blue bar chart: plants not inoculated with either the beneficial bacterium or the pathogen; purple bar chart: plants inoculated with the pathogen (Z. tritici); pink bar chart: plants inoculated with the beneficial bacterium strain BE2; green bar chart: plants inoculated with the strain BE2 and subsequently challenged with the pathogen. Statistical analyses were realized according to the Tukey test (p ≤ 0.05)
Time course of the relative expression of barley defense genes in the cultivar KWS Fantex after P. teres MUCL 28818 infection at 6, 12, 48 hpi, and 3 dpi with P. teres. Plants were inoculated with strain BE2, by immersing the pre-germinated grains in a suspension of 10.8 CFU/mL. The different experimental conditions are represented by distinct bar colors in the chart: blue bar chart: plants that were not inoculated with either the beneficial bacterium or the pathogen; purple bar chart: plants inoculated with the pathogen (P. teres); pink bar chart: plants inoculated with the beneficial bacterium strain BE2; green bar chart: plants inoculated with strain BE2 and subsequently challenged with the pathogen. Statistical analyses were realized according to the Tukey test (p ≤ 0.05)
An increase in gene expression was also highlighted in the barley assay. As shown in Fig. 9, the expression level tended to vary when plants were infected with P. teres, especially when barley plants were exposed to strain BE2. Phenylalanine ammonia-lyase (PAL2), the first enzyme in the phenylpropanoid pathway crucial for synthesizing specialized metabolites used in defense-related responses (Zhang et al. 2017), was upregulated during the initial hours after infection with P. teres. However, the expression was significantly higher when plants were bacterized with strain BE2. On the contrary, after 3 dpi, the up-regulation was maintained for the control with the pathogen, but the gene was down-regulated for the conditions with the strain BE2. In the first hours after infection, the primary response to pathogen recognition was the hypersensitive response, which leads to the release of reactive oxygen species (ROS), which induce localized cell death (Gullner et al. 2018). At 3 dpi, the GST gene was upregulated when plants were in confrontation with P. teres. This hypersensitive response could be also a result from the modification of specific plant tissues. The GSL3 gene is involve in the cell wall biosynthesis pathway, contributing to callose deposition in plant tissues (Wang et al. 2021). This gene was upregulated at 48 hpi in BE2-treated plants and challenged by the pathogen. Moreover, the pathogenesis-related proteins are considered good indicators of plant defense against fungal attacks. In this study, PR1 transcripts peaked at 48 hpi for the modality infected by P. teres, especially when they were bacterized with strain BE2. On the contrary, the PR5 gene did not show modulations, except a down-regulation at 3 dpi. Similar results were observed with LOX2, which did not show significant modifications during the initial hours when plants were infected by the pathogen, compared to the AOC gene, which was expressed in all time points. Finally, a down-regulation of nearly all genes was noticeable in the modalities inoculated with strain BE2 at 3 dpi.
Discussion
Wheat and barley are among the most widely cultivated cereal crops globally and serve vital roles in both human food and livestock feed production. According to the FAO, as of 2021, the global production of wheat was estimated at approximately 775 million tons, while barley production was estimated to be around 144 million tons. These figures rank wheat and barley as the third and fourth most produced cereal crops worldwide, respectively, trailing behind maize and rice (FAOSTAT 2022). Among the different pathogens that affect these cereals, Z. tritici and P. teres are particularly deleterious, capable of reducing yields by up to 40%. To mitigate this substantial pathogenic pressure on crops, a range of control strategies, including chemical, agronomic, and genetic methods, have been deployed (Dutilloy et al. 2022). However, in recent years, due to increased regulatory scrutiny, there has been a growing interest in new eco-friendly solutions. These include the utilization of biocontrol products and, notably, the application of beneficial bacteria, which are being considered as a better sustainable alternative (Lahlali et al. 2022).
The present study explored the capacity of the plant associated B. velezensis BE2 to protect wheat and barley from pathogens. In vitro analyses revealed that this bacterium had the ability to significantly inhibit the growth of two notable pathogens, Z. tritici and P. teres. When directly confronted, strain BE2 not only inhibited spores germination but also induced faster sporulation in the fungus. These results are in line with previous reports, which have shown that members of Bacillus can display antagonistic activity against septoria and net blotch diseases (Mejri et al. 2018; Chemitei et al. 2019; Platel et al. 2021; Dutilloy et al. 2022). The antifungal activity appears to be associated with the production of a wide range of secondary metabolites involved in biocontrol. Indeed, an in silico analysis of strain BE2’s genome pointed out the presence of many biosynthetic gene clusters involved in the synthesis of different secondary metabolites. Notably, the genome contained several gene clusters responsible for non-ribosomal peptides and polyketides, rendering strain BE2 among the Bacillus members known for producing these kinds of metabolites. These findings are in agreement with previous assays realized on Bacillus species, where several mechanisms have been identified for their efficacy against pathogens. These mechanisms encompass the synthesis of various secondary metabolites known to actively engage in direct antagonistic interactions and the induction of systemic resistance against phytopathogens (Jacobsen et al. 2004; Kildea et al. 2008; Gong et al. 2015; Dimkić et al. 2022).
Different strains of B. velezensis have received remarkable attention as promising sources of several biologically active compounds, including lipopeptides capable of suppressing phytopathogens (Rabbee et al. 2019). Our results showed that the supernatant from strain BE2 exerts a substantial inhibitory effect on both spore germination and mycelium growth of the two pathogens, even at very low concentrations. UPLC-MS analysis unveiled the production of seven distinct families of metabolites by the strain under in vitro conditions. Notably, among these metabolites, the three main families of cyclic lipopeptides including iturin, fengycin, and surfactin were identified, with their isoforms differentiated based to the length of their acyl chains (Anckaert et al. 2021). Previous studies have reported the inhibitory effect of Bacillus lipopeptides on Z. tritici (Mejri et al. 2018; Platel et al. 2021). However, to the best of our knowledge, no available information exists regarding the role of lipopeptides on P. teres.
Members of the iturin and fengycin families are mainly known for their antifungal properties affecting the cell membranes and causing the death of fungi. Iturin disturbs the cytoplasmic membrane causing K+ ions leaching and thus the membrane osmotic imbalance causing cell death. They have been reported to be effective even at low concentrations with activity on a broad spectrum of pathogens (Yu et al. 2002; Romero et al. 2007; Crane et al. 2012; Gong et al. 2015; Mejri et al. 2018). The fengycin lipopeptides interact with lipid layers of the plasma membrane and modify cell membrane permeability resulting in high concentrations of pore formation, allowing the complete efflux of intercellular contents of affected cells (Kim et al. 2004; Wang et al. 2007). The surfactin produced by the Bacillus can act synergically with iturin and fengycin and enhance the effect in the inhibition of fungal development (Mejri et al. 2018).
In addition to their antifungal properties, several lipopeptides have been described in the literature for their capacity to induce ISR during plant protection assays. The effect of cyclic lipopeptides, particularly surfactin and fengycin, in inducing ISR have been thoroughly studied in various plant-pathogen system, including Botrytis cinerea in bean and tomato (Ongena et al. 2007), Rhizoctonia solani in lettuce (Chowdhury et al. 2015), Podosphaera fusca in melon (García-Gutiérrez et al. 2013), Penicillium digitatum in citrus fruit (Waewthongrak et al. 2014), and Plasmopara viticola in grapevine (Li et al. 2019). In specific cases, iturin has also showed a similar role (Han et al. 2015; Yamamoto et al. 2015; Lam et al. 2021). Recently, several reports have highlighted the ISR mechanism mediated by cyclic lipopeptides in wheat against Z. tritici (Khong et al. 2013; Le Mire et al. 2018; Mejri et al. 2018). Additionally, surfactin has been demonstrated to stimulate biofilm formation by inducing potassium leakage in cells, which acts as a quorum-sensing signal triggering biofilm formation (López et al. 2009). Concerning the peptide/polyketide bacillaene, it plays a role in interspecies interactions (Müller et al. 2014). Recent studies have also demonstrated that bacillaene serves as an antibiotic and modulates biofilm formation, contributing to the bacterium’s protection (Li et al. 2021; Erega et al. 2021). Lastly, the production of bacillibactin siderophore was observed and confirmed with CAS assay. While many studies have highlighted correlations between siderophore production and plant growth promotion, new research suggested that siderophore can potentially serve as biocontrol agent against pathogens (Dimopoulou et al. 2021).
The beneficial bacterium strain BE2 exhibited a strong protective effect on both barley and wheat in planta. As indicated by fungal DNA detection and visual observations, the bacterium effectively reduced the development of both pathogens. However, the effectiveness of this protection depended on the mode of application. After quantifying Z. tritici, strain BE2 seemed to be more effective when applied foliar during the first days following pathogen inoculation. However, an opposite trend was observed after several days. It is suggested that strain BE2 may directly inhibit pathogen development when it comes into direct contact with the pathogen, likely through the secretion of lipopeptides. However, this effect was not long-lasting because the bacterium did not survive in the phyllosphere, as it is a rhizosphere bacterium. On the opposite, a significant and constant decease in fungal DNA was observed when drenching applications were realized to limit the development of Z. tritici or P. teres. Additionally, in case of barley protection, a significant decrease in P. teres concentration was observed from 3 dpi. It is possible to assume that strain BE2 was able to establish a close relationship with the plants roots and induce plant defense mechanisms during the first hours after infection. Strain BE2 efficiently forms biofilm, which is essential for bacteria colonization and acts as both a protective boundary and a physical barrier. This robust biofilm formation might facilitate the bacterium’s survival in the rhizosphere for several days, allowing it to establish a close symbiotic relationship with the plant, which is crucial for pathogen suppression. Moreover, the presence of the beneficial bacteria at the root level can lead to higher local concentrations of secondary metabolites, which directly enhance plant defense mechanisms (Bais et al. 2004). Consequently, the bacterium’s ability to establish a symbiotic relationship and colonize the plant may explain its effectiveness in reducing disease when applied at the root level.
In this work, several genes of interest, recognized as good markers of the induction of resistance by Z. tritici, were tracked following previous study of Samain et al. (2019). Gene expression analysis showed that when wheat and barley were treated with strain BE2 alone, all the tested genes in the wheat experiment and several in the barley assay were up-regulated at 6 hpi compared to the control. However, by 12 hpi, these genes were no longer induced when plants were treated solely with strain BE2. These results suggested that this beneficial bacterium initiated a plant defense response during the first hours after inoculation but actively blocked the immune plant response in order to establish a compatible interaction with the host. In contrast, an over-expression of these genes occurred when plants were in contact with the pathogen. Furthermore, the relative genes expression was significantly higher when plants had been previously bacterized at the root level with strain BE2, a characteristic feature of ISR and priming (Conrath et al. 2002). As a result, there was an increase in the expression of genes encoding ß-1,3-glucanase and chitinase in the first few hours after infection when the fungus adhered to the leaf surface. The expression of these two genes is crucial for wheat resistance, as they encode enzymes capable of breaking down the fungal cell wall and releasing elicitors that activate other resistance mechanisms in the plant (Ray et al. 2003; Shetty et al. 2009; Somai-Jemmali et al. 2020). Peroxidases are induced in host plant tissues after pathogen infection. These enzymes increase the production of ROS, which is one of the first responses to Z. tritici (Almagro et al. 2009). ROS acts as a toxic agent for the pathogen, limiting its spread. Additionally, peroxidases can contribute to the formation of a physical barrier, such as lignin deposition, which helps to prevent the pathogen from penetrating deeper into the plant tissue. Furthermore, in line with Shetty et al. (2003), the increased expression of the POX2 gene at 12 and 24 h after infection serves as a mechanism to curtail the development of Z. tritici during its biotrophic phase, which lasts approximately 9 to 15 days. The initial peak of expression corresponds with the early recognition of the pathogen by the host, leading to the non-specific accumulation of H2O2 at 3 and 6 h after infection. Lastly, the PR1, closely related to H2O2 and callose production, was primed in bacterized and infected conditions (Pastor et al. 2013). This protein can play an important role in resistance against Z. tritici, particularly in association with other resistance proteins linked to the phenylpropanoid and ROS pathways. The results obtained in the controlled experiments align with the results of Samain et al. (2017) and Adhikari et al. (2007), which reported that the PR1 gene serves as a reliable indicator of wheat protection against Z. tritici.
The results obtained from wheat plants exposed to strain BE2 and Z. tritici were compared with those from barley plants. Gene expression analysis revealed the upregulation of several genes associated with plant defense when confronted with P. teres, including AOC, PAL2, PR1, and GSL3. These results were in line with previous data (Backes et al. 2021a). Furthermore, the induction of the genes was more pronounced in plants that had been previously bacterized before infection with P. teres. The induction of ISR is often linked to the expression of genes involved in the synthesis of jasmonic acid (Pieterse et al. 2014), with the AOC gene being a key marker of this pathway. In this study, the expression of AOC gene was upregulated at 2 and 3 dpi when plants were infected with P. teres. However, a higher and earlier regulation was observed when plants were previously bacterized with strain BE2, indicating the ability of strain BE2 to induce a priming response in the plant during the first hours of infection when the pathogen is still in its biotic phase. To limit the proliferation of the pathogen within the leaves, plants can modulate the deposition of callose, thereby enhancing the physical barrier between infected cells and adjacent ones (Wang et al. 2021). This defense mechanism is particularly important for protecting against P. teres because this pathogen initially develops through the mesophyll without causing visible symptoms during the first hours. However, once penetration is achieved, the adjacent cells, as well as the ten contiguous cells, show an accumulation of phenolic compounds involved in mechanisms leading to programmed cell death (Lightfoot and Able 2010). The capacity of strain BE2 to induce an upregulation of GSL3 at 48 hpi, during the transition to the necrotrophy phase of the pathogen, can significantly contribute to reducing pathogen’s impact on leaves. Similar to the results in wheat assays, the expression of the PR1 gene was higher when plants were exposed to both strain BE2 and P. teres compared to plants infected with P. teres alone. Furthermore, an upregulation of the PAL gene, involved in the phenylpropanoid pathway (Backes et al. 2021a), was observed during the first hours after infection when plants were bacterized at the root level. Flavonoids, including anthocyanins, flavones, and flavonols, are plant secondary metabolites that play an important role in the plant defense response against various pathogens. Notably, low expression of the PAL gene during P. teres interaction is considered beneficial for pathogen growth and colonisation, whereas a significant increase in its expression inflicts serious damage to the pathogen (Santana Silva et al. 2020; Pandey et al. 2021). These results confirmed those obtained by Pandey et al. (2021) who showed that genes involved in pathogen stress conditions were up-regulated in susceptible and resistance barley cultivars following P. teres infection under biotic stress conditions. Additionally, there was a down-regulation of almost all genes at 3 dpi in plants exposed to strain BE2, even if they were not subsequently infected with P. teres. These observations may result from gene expression modulation by the plants when the pathogen transitions to the necrotrophy phase. Consequently, although the priming effect was less pronounced compared to wheat plants, it was evident that the beneficial bacterium can induce barley defense, especially during the first hours after infection.
In conclusion, the present study has successfully demonstrated the effectiveness of the plant-associated B. velezensis BE2 in controlling two cereal pathogens Z. tritici and P. teres, through both direct and ISR mechanisms. Through in vitro and in planta assays, we have shown that the beneficial bacterium can supress pathogen development and alleviate disease symptoms. UPLC-MS analysis has revealed the production of different families of cyclic lipopeptides by strain BE2, which may play a role in modulating plant defense genes, as previously reported (Touré et al. 2004; Ongena and Jacques 2008; Han et al. 2015; Yamamoto et al. 2015; Mejri et al. 2018). Our study suggests that the presence of strain BE2 either directly or indirectly disrupts the pathogenicity of Z. tritici and P. teres. This opens up new perspectives for utilizing B. velezensis BE2 as a biocontrol agent against septoria tritici blotch and net blotch diseases.
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary materials published online.
References
Able AJ (2003) Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 221:137–143. https://doi.org/10.1007/s00709-002-0064-1
Adhikari TB, Balaji B, Breeden J, Goodwin SB (2007) Resistance of wheat to Mycosphaerella graminicola involves early and late peaks of gene expression. Physiol Mol Plant Pathol 71:55–68. https://doi.org/10.1016/j.pmpp.2007.10.004
Albdaiwi RN, Khyami-Horani H, Ayad JY, Alananbeh KM, Al-Sayaydeh R (2019) Isolation and characterization of halotolerant plant growth promoting rhizobacteria from durum wheat (Triticum turgidum subsp. durum) cultivated in saline areas of the dead sea region. Front Microbiol 10:1639. https://doi.org/10.3389/fmicb.2019.01639
Ali S, Mir ZA, Bhat JA, Tyagi A, Chandrashekar N, Yadav P, Rawat S, Sultana M, Grover A (2018) Isolation and characterization of systemic acquired resistance marker gene PR1 and its promoter from Brassica juncea. 3 Biotech 8:10. https://doi.org/10.1007/s13205-017-1027-8
Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA (2009) Class III peroxidases in plant defence reactions. J Exp Bot 60:377–390. https://doi.org/10.1093/jxb/ern277
Anckaert A, Arias AA, Hoff G, Calonne-Salmon M, Declerck S, Ongena M (2021) The use of Bacillus spp. as bacterial biocontrol agents to control plant diseases. Microbial bioprotectants for plant disease management. Burleigh Dodds Series in Agricultural Science. Burleigh Dodds Science Publishing Limited, pp 247–300. https://doi.org/10.19103/as.2021.0093.10
Andrić S, Rigolet A, Argüelles Arias A, Steels S, Hoff G, Balleux G, Ongena L, Höfte M, Meyer T, Ongena M (2023) Plant-associated Bacillus mobilizes its secondary metabolites upon perception of the siderophore pyochelin produced by a Pseudomonas competitor. ISME J 17:263–75. https://doi.org/10.1038/s41396-022-01337-1
Backes A, Charton S, Planchon S, Esmaeel Q, Sergeant K, Hausman J-F, Renaut J, Barka EA, Jacquard C, Guerriero G (2021a) Gene expression and metabolite analysis in barley inoculated with net blotch fungus and plant growth-promoting rhizobacteria. Plant Physiol Biochem PPB 168:488–500. https://doi.org/10.1016/j.plaphy.2021.10.027
Backes A, Guerriero G, Ait Barka E, Jacquard C (2021b) Pyrenophora teres: taxonomy, morphology, interaction with barley, and mode of control. Front Plant Sci 12:614951. https://doi.org/10.3389/fpls.2021.614951
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319. https://doi.org/10.1104/pp.103.028712
Bearchell SJ, Fraaije BA, Shaw MW, Fitt BDL (2005) Wheat archive links long-term fungal pathogen population dynamics to air pollution. Proc Natl Acad Sci USA 102:5438–5442. https://doi.org/10.1073/pnas.0501596102
Blin K, Shaw S, Kautsar SA, Medema MH, Weber T (2021) The antiSMASH database version 3: increased taxonomic coverage and new query features for modular enzymes. Nucleic Acids Res 49:D639–D643. https://doi.org/10.1093/nar/gkaa978
Brokenshire T (2007) Wheat debris as an inoculum source for seedling infection by Septoria tritici. Plant Pathol 24:202–207. https://doi.org/10.1111/j.1365-3059.1975.tb01895.x
Carlsen SA, Neupane A, Wyatt NA, Richards JK, Faris JD, Xu SS, Brueggeman RS, Friesen TL (2017) Characterizing the Pyrenophora teres f maculata–barley interaction using pathogen genetics. G3 GenesGenomesGenetics 7:2615–2626. https://doi.org/10.1534/g3.117.043265
Chemitei K, Amendi MB, Mwamburi LA, Ochuodho JO (2019) Bio-control of net-blotch and scald pathogens of barley using Paenibacillus polymyxa KAI245 isolated from sorghum rhizosphere in western Kenya. Am J Microbiol Res 7:28–36. https://doi.org/10.12691/ajmr-7-1-5
Chowdhury SP, Hartmann A, Gao X, Borriss R (2015) Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 – a review. Front Microbiol 6:780. https://doi.org/10.3389/fmicb.2015.00780
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959. https://doi.org/10.1128/AEM.71.9.4951-4959.2005
Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant–pathogen interactions. Trends Plant Sci 7:210–216. https://doi.org/10.1016/S1360-1385(02)02244-6
Crane J, Gibson D, Vaughan R, Bergstrom G (2012) Iturin levels on wheat spikes linked to biological control of fusarium head blight by Bacillus amyloliquefaciens. Phytopathology 103:146–155. https://doi.org/10.1094/PHYTO-07-12-0154-R
Decouard B, Bailly M, Rigault M, Marmagne A, Arkoun M, Soulay F, Caïus J, Paysant-Le Roux C, Louahlia S, Jacquard C, Esmaeel Q, Chardon F, Masclaux-Daubresse C, Dellagi A (2022) Genotypic variation of nitrogen use efficiency and amino acid metabolism in barley. Front Plant Sci 12:807798. https://doi.org/10.3389/fpls.2021.807798
Dimkić I, Janakiev T, Petrović M, Degrassi G, Fira D (2022) Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms - a review. Physiol Mol Plant Pathol 117:101754. https://doi.org/10.1016/j.pmpp.2021.101754
Dimopoulou A, Theologidis I, Benaki D, Koukounia M, Zervakou A, Tzima A, Diallinas G, Hatzinikolaou DG, Skandalis N (2021) Direct antibiotic activity of bacillibactin broadens the biocontrol range of Bacillus amyloliquefaciens MBI600. MSphere 6:e00376-21. https://doi.org/10.1128/mSphere.00376-21
Dutilloy E, Oni FE, Esmaeel Q, Clément C, Barka EA (2022) Plant beneficial bacteria as bioprotectants against wheat and barley diseases. J Fungi 8:632. https://doi.org/10.3390/jof8060632
El Arbi A, Rochex A, Chataigné G, Béchet M, Lecouturier D, Arnauld S, Gharsallah N, Jacques P (2016) The Tunisian oasis ecosystem is a source of antagonistic Bacillus spp. producing diverse antifungal lipopeptides. Res Microbiol 167:46–57. https://doi.org/10.1016/j.resmic.2015.09.003
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490. https://doi.org/10.3389/fmicb.2017.01490
Erega A, Stefanic P, Dogsa I, Danevčič T, Simunovic K, Klančnik A, Smole Možina S, Mandic Mulec I (2021) Bacillaene mediates the inhibitory effect of Bacillus subtilis on Campylobacter jejuni biofilms. Appl Environ Microbiol 87:e0295520. https://doi.org/10.1128/AEM.02955-20
Esmaeel Q, Jacquard C, Sanchez L, Clément C, Barka EA (2020) The mode of action of plant associated Burkholderia against grey mould disease in grapevine revealed through traits and genomic analyses. Sci Rep 10:19393. https://doi.org/10.1038/s41598-020-76483-7
Esmaeel Q, Pupin M, Jacques P, Leclère V (2018) Nonribosomal peptides and polyketides of Burkholderia: new compounds potentially implicated in biocontrol and pharmaceuticals. Environ Sci Pollut Res 25:29794–29807. https://doi.org/10.1007/s11356-017-9166-3
Esmaeel Q, Pupin M, Kieu NP, Chataigné G, Béchet M, Deravel J, Krier F, Höfte M, Jacques P, Leclère V (2016) Burkholderia genome mining for nonribosomal peptide synthetases reveals a great potential for novel siderophores and lipopeptides synthesis. MicrobiologyOpen 5:512–526. https://doi.org/10.1002/mbo3.347
FAOSTAT (2022) Food and agriculture organization of the United Nations. https://www.fao.org/faostat/en/#data/RP. Retrieved from https://www.fao.org/3/cc2211en/cc2211en.pdf. Accessed 19 Apr 2022
Fraaije BA, Lovell DJ, Rohel EA, Hollomon DW (1999) Rapid detection and diagnosis of Septoria tritici epidemics in wheat using a polymerase chain reaction/PicoGreen assay. J Appl Microbiol 86:701–708. https://doi.org/10.1046/j.1365-2672.1999.00716.x
García-Gutiérrez L, Zeriouh H, Romero D, Cubero J, de Vicente A, Pérez-García A (2013) The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate- and salicylic acid-dependent defence responses. Microb Biotechnol 6:264–274. https://doi.org/10.1111/1751-7915.12028
Ghaffariyan S, Mohammadi SA, Aharizad S (2012) DNA isolation protocol for the medicinal plant lemon balm (Melissa officinalis, Lamiaceae). Genet Mol Res GMR 11:1049–1057. https://doi.org/10.4238/2012.April.27.3
Gong A-D, Li H-P, Yuan Q-S, Song X-S, Yao W, He W-J, Zhang J-B, Liao Y-C (2015) Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76–3 from wheat spikes against Fusarium graminearum. PLoS ONE 10:e0116871. https://doi.org/10.1371/journal.pone.0116871
Gullner G, Komives T, Király L, Schröder P (2018) Glutathione S-transferase enzymes in plant-pathogen interactions. Front Plant Sci 9:1836. https://doi.org/10.3389/fpls.2018.01836
Han Q, Wu F, Wang X, Qi H, Shi L, Ren A, Liu Q, Zhao M, Tang C (2015) The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ Microbiol 17:1166–1188. https://doi.org/10.1111/1462-2920.12538
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev MMBR 79:293–320. https://doi.org/10.1128/MMBR.00050-14
Issa A, Esmaeel Q, Sanchez L, Courteaux B, Guise J-F, Gibon Y, Ballias P, Clément C, Jacquard C, Vaillant-Gaveau N, AïtBarka E (2018) Impacts of Paraburkholderia phytofirmans strain PsJN on tomato (Lycopersicon esculentum L.) under high temperature. Front Plant Sci. 9:1397. https://doi.org/10.3389/fpls.2018.01397
Jacobsen BJ, Zidack NK, Larson BJ (2004) The role of Bacillus -based biological control agents in integrated pest management systems: plant diseases. Phytopathology 94:1272–1275. https://doi.org/10.1094/PHYTO.2004.94.11.1272
Khong N, Randoux B, Deravel J, Tisserant B, Tayeh C, Coutte F, Bourdon N, Jacques P, Reignault P (2013) Induction of resistance in wheat by bacterial cyclic lipopeptides. Commun Agric Appl Biol Sci 78:479–487
Kildea S, Ransbotyn V, Khan MR, Fagan B, Leonard G, Mullins E, Doohan FM (2008) Bacillus megaterium shows potential for the biocontrol of septoria tritici blotch of wheat. Biol Control 47:37–45. https://doi.org/10.1016/j.biocontrol.2008.07.001
Kim PI, Bai H, Bai D, Chae H, Chung S, Kim Y, Park R, Chi Y-T (2004) Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J Appl Microbiol 97:942–949. https://doi.org/10.1111/j.1365-2672.2004.02356.x
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, Belabess Z, Barka EA (2022) Biological control of plant pathogens: a global perspective. Microorganisms 10:596. https://doi.org/10.3390/microorganisms10030596
Lam VB, Meyer T, Arias AA, Ongena M, Oni FE, Höfte M (2021) Bacillus cyclic lipopeptides iturin and fengycin control rice blast caused by Pyricularia oryzae in potting and acid sulfate soils by direct antagonism and induced systemic resistance. Microorganisms 9:1441. https://doi.org/10.3390/microorganisms9071441
Landy M, Warren GH, RosenmanM SB, Colio LG (1948) Bacillomycin: an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med 67:539–541. https://doi.org/10.3181/00379727-67-16367
Le Mire G, Siah A, Brisset M-N, Gaucher M, Deleu M, Jijakli MH (2018) Surfactin protects wheat against Zymoseptoria tritici and activates both salicylic acid- and jasmonic acid-dependent defense responses. Agriculture 8:11. https://doi.org/10.3390/agriculture8010011
Leclère V, Béchet M, Adam A, Guez J-S, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M, Jacques P (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol 71:4577–4584. https://doi.org/10.1128/AEM.71.8.4577-4584.2005
Leisova L, Minarikova V, Kucera L, Ovesna J (2006) Quantification of Pyrenophora teres in infected barley leaves using real-time PCR. J Microbiol Methods 67:446–455. https://doi.org/10.1016/j.mimet.2006.04.018
Li H, Han X, Dong Y, Xu S, Chen C, Feng Y, Cui Q, Li W (2021) Bacillaenes: decomposition trigger point and biofilm enhancement in Bacillus. ACS Omega 6:1093–1098. https://doi.org/10.1021/acsomega.0c03389
Li Y, Héloir M-C, Zhang X, Geissler M, Trouvelot S, Jacquens L, Henkel M, Su X, Fang X, Wang Q, Adrian M (2019) Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol Plant Pathol 20:1037–1050. https://doi.org/10.1111/mpp.12809
Lightfoot D, Able A (2010) Growth of Pyrenophora teres in planta during barley net blotch disease. Aust Plant Pathol 39:499–507. https://doi.org/10.1071/AP10121
Lightfoot DJ, Mcgrann GRD, Able AJ (2017) The role of a cytosolic superoxide dismutase in barley–pathogen interactions. Mol Plant Pathol 18:323–335. https://doi.org/10.1111/mpp.12399
Liu Z, Ellwood SR, Oliver RP, Friesen TL (2011) Pyrenophora teres: profile of an increasingly damaging barley pathogen. Mol Plant Pathol 12:1–19. https://doi.org/10.1111/j.1364-3703.2010.00649.x
López D, Fischbach MA, Chu F, Losick R, Kolter R (2009) Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci 106:280–285. https://doi.org/10.1073/pnas.0810940106
Lovell DJ, Hunter T, Powers SJ, Parker SR, Van den Bosch F (2004) Effect of temperature on latent period of septoria leaf blotch on winter wheat under outdoor conditions. Plant Pathol 53:170–181. https://doi.org/10.1111/j.0032-0862.2004.00983.x
Mejri S, Siah A, Coutte F, Magnin-Robert M, Randoux B, Tisserant B, Krier F, Jacques P, Reignault P, Halama P (2018) Biocontrol of the wheat pathogen Zymoseptoria tritici using cyclic lipopeptides from Bacillus subtilis. Environ Sci Pollut Res Int 25:29822–29833. https://doi.org/10.1007/s11356-017-9241-9
Morin L (2020) Progress in biological control of weeds with plant pathogens. Annu Rev Phytopathol 58:201–223. https://doi.org/10.1146/annurev-phyto-010820-012823
Müller S, Strack SN, Hoefler BC, Straight PD, Kearns DB, Kirby JR (2014) Bacillaene and sporulation protect Bacillus subtilis from predation by Myxococcus xanthus. Appl Environ Microbiol 80:5603–5610. https://doi.org/10.1128/AEM.01621-14
Olson RD, Assaf R, Brettin T, Conrad N, Cucinell C, Davis JJ, Dempsey DM, Dickerman A, Dietrich EM, Kenyon RW, Kuscuoglu M, Lefkowitz EJ, Lu J, Machi D, Macken C, Mao C, Niewiadomska A, Nguyen M, Olsen GJ, Overbeek JC, Parrello B, Parrello V, Porter JS, Pusch GD, Shukla M, Singh I, Stewart L, Tan G, Thomas C, VanOeffelen M, Vonstein V, Wallace ZS, Warren AS, Wattam AR, Xia F, Yoo H, Zhang Y, Zmasek CM, Scheuermann RH, Stevens RL (2023) Introducing the bacterial and viral bioinformatics resource center (BV-BRC): a resource combining PATRIC, IRD and ViPR. Nucleic Acids Res 51:D678–D689. https://doi.org/10.1093/nar/gkac1003
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. https://doi.org/10.1016/j.tim.2007.12.009
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny J-L, Thonart P (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 9:1084–1090. https://doi.org/10.1111/j.1462-2920.2006.01202.x
Palma-Guerrero J, Ma X, Torriani SFF, Zala M, Francisco CS, Hartmann FE, Croll D, McDonald BA (2017) Comparative transcriptome analyses in Zymoseptoria tritici reveal significant differences in gene expression among strains during plant infection. Mol Plant Microbe Interact 30:231–244. https://doi.org/10.1094/MPMI-07-16-0146-R
Pandey C, Großkinsky DK, Westergaard JC, Jørgensen HJL, Svensgaard J, Christensen S, Schulz A, Roitsch T (2021) Identification of a bio-signature for barley resistance against Pyrenophora teres infection based on physiological, molecular and sensor-based phenotyping. Plant Sci Int J Exp Plant Biol 313:111072. https://doi.org/10.1016/j.plantsci.2021.111072
Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V (2013) Primed plants do not forget. Environ Exp Bot 94:46–56. https://doi.org/10.1016/j.envexpbot.2012.02.013
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375. https://doi.org/10.1146/annurev-phyto-082712-102340
Platel R, Sawicki M, Esmaeel Q, Randoux B, Trapet P, El Guilli M, Chtaina N, Arnauld S, Bricout A, Rochex A, Facon N, Halama P, Jacquard C, Ait Barka E, Reignault P, Magnin-Robert M, Siah A (2021) Isolation and identification of lipopeptide-producing Bacillus velezensis strains from wheat phyllosphere with antifungal activity against the wheat pathogen Zymoseptoria tritici. Agronomy 12:95. https://doi.org/10.3390/agronomy12010095
Rabbee MF, Ali MS, Choi J, Hwang BS, Jeong SC, Baek K (2019) Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules 24:1046. https://doi.org/10.3390/molecules24061046
Ray S, Anderson JM, Urmeev FI, Goodwin SB (2003) Rapid induction of a protein disulfide isomerase and defense-related genes in wheat in response to the hemibiotrophic fungal pathogen Mycosphaerella graminicola. Plant Mol Biol 53:741–754. https://doi.org/10.1023/B:PLAN.0000019120.74610.52
Rekha K, Baskar B, Srinath S, Usha B (2018) Plant-growth-promoting rhizobacteria Bacillus subtilis RR4 isolated from rice rhizosphere induces malic acid biosynthesis in rice roots. Can J Microbiol 64:20–27. https://doi.org/10.1139/cjm-2017-0409
Romero D, de Vicente A, Rakotoaly RH, Dufour SE, Veening J-W, Arrebola E, Cazorla FM, Kuipers OP, Paquot M, Pérez-García A (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant Microbe Interact 20:430–440. https://doi.org/10.1094/MPMI-20-4-0430
Rondeau M, Esmaeel Q, Crouzet J, Blin P, Gosselin I, Sarazin C, Pernes M, Beaugrand J, Wisniewski-Dyé F, Vial L, Faure D, Clément C, Ait Barka E, Jacquard C, Sanchez L (2019) Biofilm-constructing variants of Paraburkholderia phytofirmans PsJN Outcompete the wild-type form in free-living and static conditions but not in planta. Appl Environ Microbiol 85:e02670-e2718. https://doi.org/10.1128/AEM.02670-18
Rupp S, Weber RWS, Rieger D, Detzel P, Hahn M (2017) Spread of Botrytis cinerea strains with multiple fungicide resistance in German horticulture. Front Microbiol 7:2075. https://doi.org/10.3389/fmicb.2016.02075
Sabir A, Yazici MA, Kara Z, Sahin F (2012) Growth and mineral acquisition response of grapevine rootstocks (Vitis spp.) to inoculation with different strains of plant growth-promoting rhizobacteria (PGPR). J Sci Food Agric 92:2148–2153. https://doi.org/10.1002/jsfa.5600
Samain E, Aussenac T, Selim S (2019) The effect of plant genotype, growth stage, and Mycosphaerella graminicola strains on the efficiency and durability of wheat-induced resistance by Paenibacillus sp. strain B2. Front Plant Sci 10:587. https://doi.org/10.3389/fpls.2019.00587
Samain E, Ernenwein C, Aussenac T, Selim S (2022) Effective and durable systemic wheat-induced resistance by a plant-growth-promoting rhizobacteria consortium of Paenibacillus sp. strain B2 and Arthrobacter spp. strain AA against Zymoseptoria tritici and drought stress. Physiol Mol Plant Pathol 119:101830. https://doi.org/10.1016/j.pmpp.2022.101830
Samain E, Van Tuinen D, Jeandet D, Aussenac T, Selim S (2017) Biological control of septoria leaf blotch and growth promotion in wheat by Paenibacillus sp. strain B2 and Curtobacterium plantarum strain EDS. Biol Control 114:87–96. https://doi.org/10.1016/j.biocontrol.2017.07.012
Santana Silva RJ, Alves RM, Peres Gramacho K, Marcellino LH, Micheli F (2020) Involvement of structurally distinct cupuassu chitinases and osmotin in plant resistance to the fungus Moniliophthora perniciosa. Plant Physiol Biochem 148:142–151. https://doi.org/10.1016/j.plaphy.2020.01.009
Selim S, Roisin-Fichter C, Andry J-B, Bogdanow B, Sambou R (2014) Real-time PCR to study the effect of timing and persistence of fungicide application and wheat varietal resistance on Mycosphaerella graminicola and its sterol 14α-demethylation-inhibitor-resistant genotypes. Pest Manag Sci 70:60–69. https://doi.org/10.1002/ps.3525
Shetty NP, Jensen JD, Knudsen A, Finnie C, Geshi N, Blennow A, Collinge DB, Jørgensen HJL (2009) Effects of beta-1,3-glucan from Septoria tritici on structural defence responses in wheat. J Exp Bot 60:4287–4300. https://doi.org/10.1093/jxb/erp269
Shetty NP, Kristensen BK, Newman M-A, Møller K, Gregersen PL, Jørgensen HJL (2003) Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol Mol Plant Pathol 62:333–346. https://doi.org/10.1016/S0885-5765(03)00079-1
Shetty NP, Mehrabi R, Lütken H, Haldrup A, Kema GHJ, Collinge DB, Jørgensen HJL (2007) Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol 174:637–647. https://doi.org/10.1111/j.1469-8137.2007.02026.x
Shewry PR (2009) Wheat. J Exp Bot 60:1537–1553. https://doi.org/10.1093/jxb/erp058
Somai-Jemmali L, Siah A, Randoux B, Magnin-Robert M, Halama P, Hamada W, Reignault Ph (2020) Brown alga Ascophyllum nodosum extract-based product, Dalgin Active®, triggers defense mechanisms and confers protection in both bread and durum wheat against Zymoseptoria tritici. J Appl Phycol 32:3387–3399. https://doi.org/10.1007/s10811-020-02200-6
Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378. https://doi.org/10.1104/pp.106.079467
Touré Y, Ongena M, Jacques P, Guiro A, Thonart P (2004) Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J Appl Microbiol 96:1151–1160. https://doi.org/10.1111/j.1365-2672.2004.02252.x
Villena J, Kitazawa H, Van Wees SCM, Pieterse CMJ, Takahashi H (2018) Receptors and signaling pathways for recognition of bacteria in livestock and crops: prospects for beneficial microbes in healthy growth strategies. Front Immunol 9:2223. https://doi.org/10.3389/fimmu.2018.02223
Waewthongrak W, Leelasuphakul W, McCollum G (2014) Cyclic lipopeptides from Bacillus subtilis ABS–S14 elicit defense-related gene expression in citrus fruit. PLoS ONE 9:e109386. https://doi.org/10.1371/journal.pone.0109386
Wang J, Liu J, Chen H, Yao J (2007) Characterization of Fusarium graminearum inhibitory lipopeptide from Bacillus subtilis IB. Appl Microbiol Biotechnol 76:889–894. https://doi.org/10.1007/s00253-007-1054-1
Wang Y, Li X, Fan B, Zhu C, Chen Z (2021) Regulation and function of defense-related callose deposition in Plants. Int J Mol Sci 22:2393. https://doi.org/10.3390/ijms22052393
Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey M-D, AsyrafMd Hatta M, Hinchliffe A, Steed A, Reynolds D, Adamski NM, Breakspear A, Korolev A, Rayner T, Dixon LE, Riaz A, Martin W, Ryan M, Edwards D, Batley J, Raman H, Carter J, Rogers C, Domoney C, Moore G, Harwood W, Nicholson P, Dieters MJ, DeLacy IH, Zhou J, Uauy C, Boden SA, Park RF, Wulff BBH, Hickey LT (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nat Plants 4:23–29. https://doi.org/10.1038/s41477-017-0083-8
Yamamoto S, Shiraishi S, Suzuki S (2015) Are cyclic lipopeptides produced by Bacillus amyloliquefaciens S13–3 responsible for the plant defence response in strawberry against Colletotrichum gloeosporioides? Lett Appl Microbiol 60:379–386. https://doi.org/10.1111/lam.12382
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963. https://doi.org/10.1016/S0038-0717(02)00027-5
Zhang C, Wang X, Zhang F, Dong L, Wu J, Cheng Q, Qi D, Yan X, Jiang L, Fan S, Li N, Li D, Xu P, Zhang S (2017) Phenylalanine ammonia-lyase2.1 contributes to the soybean response towards Phytophthora sojae infection. Sci Rep 7:7242. https://doi.org/10.1038/s41598-017-07832-2
Acknowledgements
The authors are very grateful to le Groupe Soufflet for providing the wheat and barley cultivars used in the study.
Funding
This work was supported by the University of Reims Champagne-Ardenne. We gratefully acknowledge the financial support provided by the Bio4Grain project, ADEME (Agence de la Transition Ecologique), France.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.D, EAB, C.C, and Q.E; methodology E.D, S.S, A.A.A, N.R, J.F.G, and Q.E; software E.D, A.A.A, N.R, and Q.E; validation E.D; formal analysis E.D, A.A.A, N.R, and Q.E; investigation E.D, E.A.B, and Q.E; Writing—Original draft preparation E.D and Q.E. Writing—Review and editing E.D, A.A.A, N.R, J.F.G, M.D, V.L, S.S, C.J, P.J, C.C, E.A.B, and Q.E. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutilloy, E., Arias, A.A., Richet, N. et al. Bacillus velezensis BE2 controls wheat and barley diseases by direct antagonism and induced systemic resistance. Appl Microbiol Biotechnol 108, 64 (2024). https://doi.org/10.1007/s00253-023-12864-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12864-y